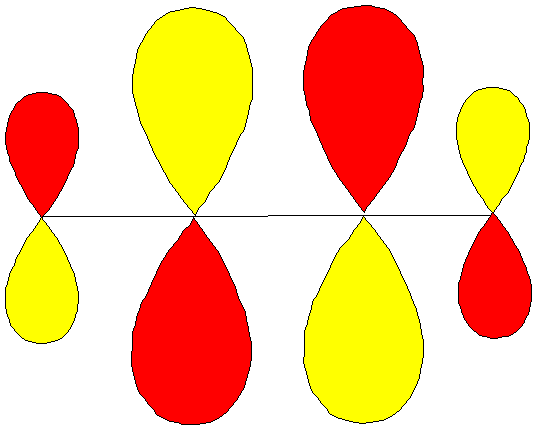
The Hückel p molecular orbitals of 1,3-butadiene
What do the
orbitals look like?
(Atkins pg.
436)
· Draw the orbitals using the coefficients c1, c2, c3 and c4 for each molecular orbital in turn.
· Both the sign and the size of the coefficient for each atomic (2p) orbital are important
· Diagrams here drawn to emphasize nodes (sign changes), size isn’t important for this
y1 -- lowest
energy p M.O. (E = a + 1.62b)
-- no nodes other than in molecular plane:
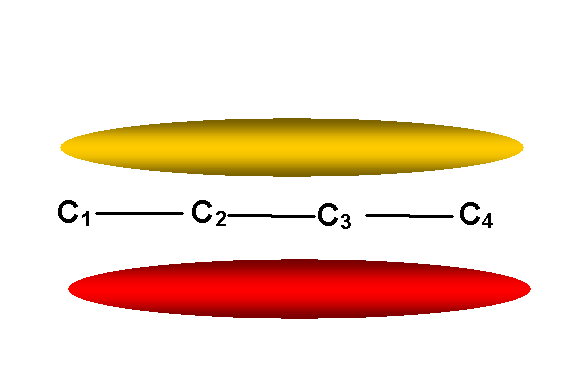
y1 = 0.37f1 + 0.6f2 + 0.6f3 + 0.37f4
_______________________________________________
y2 -- second
lowest energy p MO (E = a + 0.62b)
-- one node other than in molecular plane:
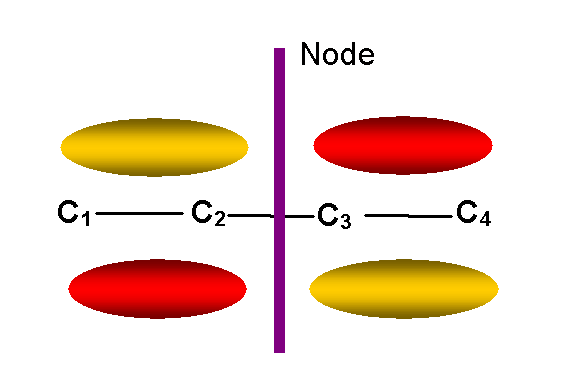
y2 = 0.6f1 + 0.37f2 – 0.37f3 – 0.6f4
_______________________________________________
y3 -- first
unoccupied antibonding p MO (E = a – 0.62b)
-- two nodes other than in molecular plane:
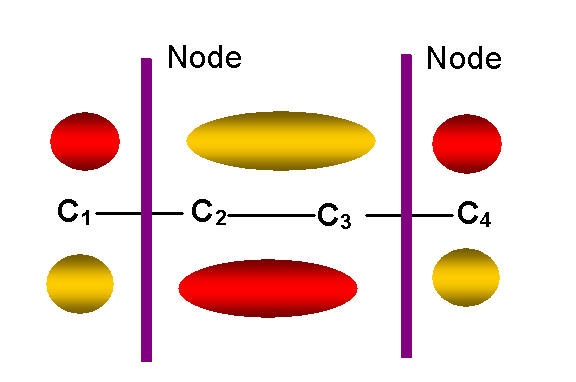
y3 = 0.6f1 – 0.37f2 – 0.37f3 + 0.6f4
_______________________________________________
y4 -- highest
antibonding p MO (E = a – 1.62b)
-- 3 nodes other than in molecular plane:

y4 = 0.37f1 – 0.6f2 + 0.6f3 + 0.37f4
Or, when size matters: