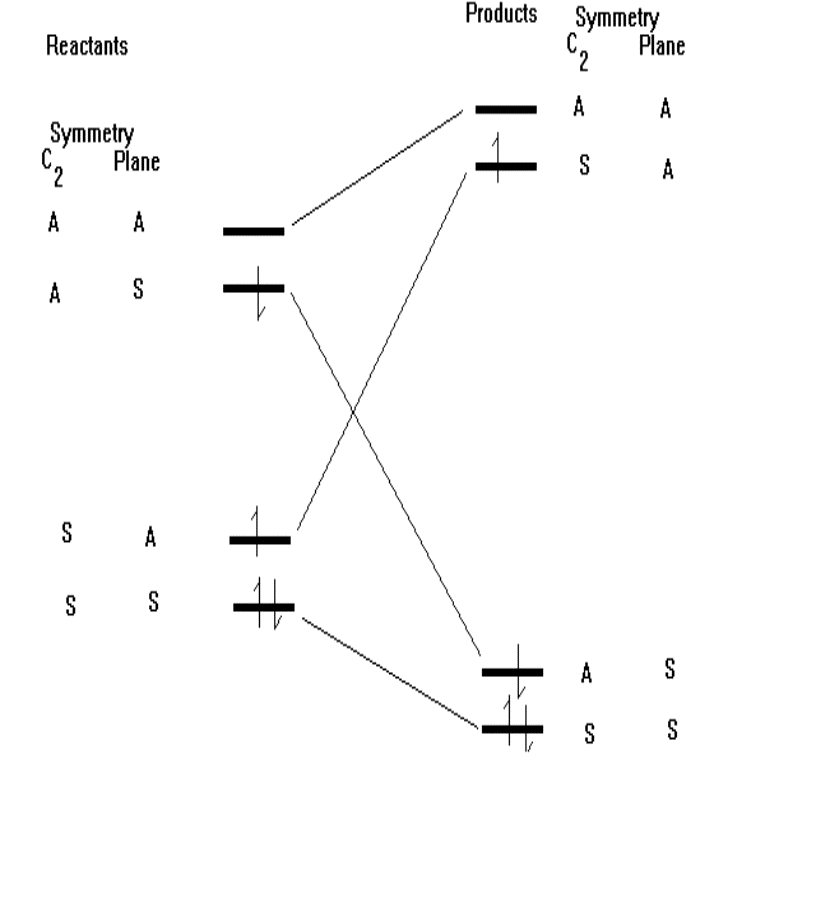
Photon-assisted cycloaddition
·
We
have seen that the thermal cycloaddition of two ethenes is “forbidden” because
the energy of the reactants goes up significantly as the reactants (two
ethenes) approach each other.
·
We reach this conclusion through the use of the correlation
diagram, which correlates or joins up orbitals of the reactants and products
which have the same symmetry.
·
From
the fact that the HOMO goes up in energy and the energy of the reactants
increases as the reaction proceeds the conclusion is reached that the reaction
has a substantial barrier to reactions and is therefore “forbidden”.
How can we change this situation?
·
We can rearrange the situation so that the reaction does not
increase as the reaction proceeds.
·
It is possible to do this by exciting the electrons in the
reactants using UV light.
The correlation diagram then looks like: