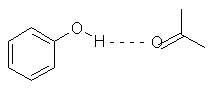
All biological structures have three fundamental noncovalent interactions, these being:
Electrostatic bonds - where a charged group on a substrate can attract an oppositely charged group on an enzyme, and this is given by Coulomb's Law:
F = q1q2 / r2D
The charges are represented by q 1 and q 2 are just the charges of the two groups, r is the distance between them, and D is the dielectric constant. Hydrogen Bonds - can be formed between charged or uncharged molecules. H- bonds are much stronger than VDW bonds, but comparatively weaker than covalent bonds.
