
Conformational changes of proteins occur when a protein is bound to another molecule, i.e. a substrate. The functioning of the immune system relies heavily on the binding of foreign molecules to antibody protein molecules . Proteins ability to interact on specific binding sites of varying molecules is due to their formation of complementary surfaces, meaning positive and negative charges on residues, and clefts, for example lock and key model.
Enzymes are biological catalysts, and play an important role in metabolism. Organisms need catalysts to make sure that the rate of metabolic activity is at the correct speed. They are highly effective at catalyzing a large range of chemical reactions, due to their ability to bind at specific sites on a wide range of molecules. Reaction catalyses involves the stabilizing of the transition state in a reaction profile pathway. Enzymes can interact and couple the reaction of several binding sites, which results in molecular switching of catalytic activity. Enzymes revolutionize chemical reaction without suffering any change themselves, they are unaltered.
Well how do enzymes work?
they firstly attract and stick to the reacting molecules, hence making it easier for them to meet. Secondly, they provide an alternative route in helping to lower the activation barrier of the reaction, so that a greater proportion of collisions of enough energy to result in a chemical reaction. The correct orientation of the reacting molecules is also important such that the reactive groups are nearer to each other. So what make enzymes special is its specificity, this can be illustrated as follows:

This is an induced fit model where the active site of the enzyme wraps around the substrate molecule. The enzyme changes shape upon binding substrate. the active site has a shape complimentary to that of the substrate, only after the substrate is bound.
In order for an enzyme and substrate to bind they need to physically fit, and in order to stay fit, they need to keep their attractive forces in place. One the enzyme and substrate have bound together, they form and enzyme/substrate complex. The binding process can be so selective that the enzyme can discriminate between two enantiomers of a chiral substrate. Also it is misinterpreted that the active site has a fixed shape, and this is certainly not true. The induced fit model shown above (which was developed by Daniel E. Koshland in 1958), has the wall of its active site (cleft) move a little to wrap around the substrate as it binds. This type of flexing induces strain on the active site and substrate, which in turn helps to lower the activation energy of the reaction.
A another example of enzyme - substrate bound complex:
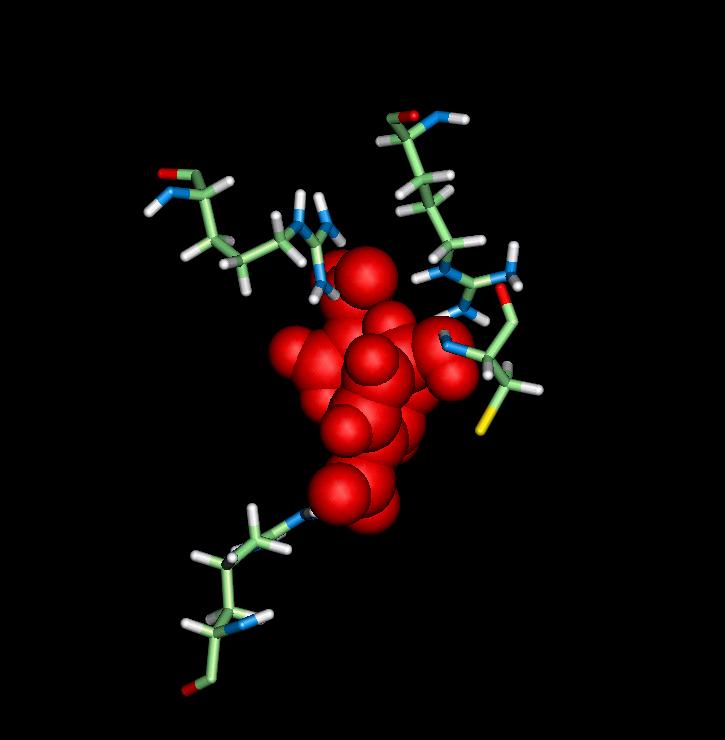
The above complex shows the important residues of the enzyme Chorismate Mutase, being Arg62, Arg6, and Cys74, that play an important role in catalytic activity, by stabilizing the transition state.
Next topic follows the mechanisms of enzyme-catalyzed reactions.