![]()
![]()
This proposal was fully funded by the EPSRC. The original objectives of this proposal can be summarised as:
Most of the above objectives were achieved, with the resulting
publication of 6 papers in refereed scientific journals. The
grant from the EPSRC Materials Commission was relatively small
(£63k) but provided funds to support a project studentship
(Stuart Leeds) for 3 years, and for the purchase of some equipment
and accessories necessary to allow the mass spectrometer to be
coupled to the microwave reactor.
(i) Assembly and construction of the MBMS-MW reactor
This was the most problematic and time-consuming part of the project. The system involved a specially designed Astex-type microwave reactor, working at 1 kW, with a heated substrate stage and electronic process control and safety interlocks. The chamber is non-standard, in that it has a 6"-diameter port in the side to allow the mass spectrometer probe to access the plasma. This port altered the cylindrical symmetry of the chamber, and caused problems with the stability of the microwave plasma ball, which often jumped to the window or formed unwanted secondary plasmas around the port opening. This was eventually solved by redesigning the entire substrate heater assembly to make the gap between the substrate platen and the chamber wall much larger, so preventing the formation of secondary plasmas.
The existing MBMS system had been previously used on a hot filament reactor, and so was known to be reasonably reliable. However we encountered a number of problems with the sampling cone used to extract gas from the centre of the plasma ball. Previously, we had used a stainless steel cone with a 100 mm-diameter orifice, and this worked extremely well in the filament reactor. However, in the MW reactor, the entire cone is immersed within the plasma ball, and we found that the steel reacted with gas phase carbon species to produce large amounts of graphite, which affected growth rates, purity and reproducibility of the diamond film. An alternative cone made from quartz was then attempted, but this rapidly etched in the hot H-plasma. Finally a cone made from molybdenum was used. Due to the difficulties with machining accurate holes in Mo, the 100 mm orifice was drilled using a Nd:YAG laser.
The finished apparatus is shown in Figs.1. and 2. Due to the extreme complexity of the system, it took nearly 18 months before the whole apparatus was working reliably enough to perform repeatable measurements, and unfortunately, this delay reduced the number of experiments we were able to perform within the 3 year time-scale of the PhD grant.
(ii) Deposition of good quality diamond filmsThe MW reactor is now routinely used to deposit high quality diamond films on Si (100) and Mo substrates. Examples of films produced in this reactor are shown in Figs.3 and 4, using CH4/H2 gas mixtures, with and without addition of N2, respectively. Film quality was monitored using laser Raman spectroscopy and a variety of laser excitation wavelengths [1]. When performing these measurements we found that the intensities of the diamond and non-diamond components in the Raman spectra vary with the wavelength of the laser excitation. This showed that laser Raman at different wavelengths can be used as a selective probe for the different constituents of the deposited film. Using this 1 kW microwave system, growth rates of up to 2 mm h-1 have been achieved, and films deposited in this reactor have been used to aid the research of a number of groups, both at Bristol [2] and elsewhere [3]. The facility to post-process CVD diamond films, e.g. perform hydrogen passivation and create surface H termination, has also been used successfully to change the electronic and field emission properties of diamond films [4]. The limited number of bias enhanced nucleation experiments attempted thus far have been less successful, mainly due to the problems associated with a low deposition rate (because oriented growth is normally only observed after a diamond thickness of about 100 mm, and this was difficult to attain in a reasonable time-scale using our low power system).
(iii) MBMS experiments
After solving the numerous problems mentioned above, the MBMS system was used to obtain quantitative mole fractions for all the important gas phase species present during diamond growth for a number of different process gas mixtures. The aim was to repeat some aspects of our previous work using the hot filament reactor, allowing us to compare directly the chemistry occurring within the MW reactor to that seen in the HF system. To do this we ensured that we sampled the gas from the plasma at a position which gave equivalent growth conditions to the experiments performed on the HF system. This proved successful, with detailed analyses of the gas phase chemistry in the chamber when using different hydrocarbon gases in H2 (CH4, C2H6, C2H4, C2H2) revealing that the cracking patterns and mole fraction ratios of the major species were independent of reactor type or activation method [5]. This is important, because it confirms that CVD diamond grows by a mechanism that is common to both types of reactor, since both reactors result in very similar gas phase composition and film growth rates. Quantitative mole fractions were obtained for a variety of different process conditions, including CH4, C2H2, C2H4 and C2H6 in H2 (C:H ratio maintained at 1%). An example of the data produced in these experiments is shown in Fig.5. Note that the total carbon balance, which is the sum of the mole fractions of all the carbon containing species, decreases with microwave power, and hence gas temperature. This is a thermal diffusion effect, caused by steep temperature gradients near the plasma edge.
To further compare with the results from the HF reactor, MBMS measurements were made from H2/CH4 with the addition of nitrogen-containing gases, such as N2, NH3, CH3NH2 and HCN. Again, detailed, quantitative measurements were obtained of the gas phase composition for a variety of different process conditions [6]. The main findings are that if N is added in a reactive form (such as NH3, or CH3NH2), it will rapidly react with any C-containing species within the plasma to form stable HCN. Thus, the C is 'locked-up' as a gas phase species and is not free to deposit onto the substrate as diamond. However, if N is added as N2 molecules, then the (1 kW) plasma is insufficiently energetic to break apart significant numbers of the strong N-N bonds. Thus, N2 acts as a 'spectator' molecule in the gas phase chemistry. Only at higher MW powers do we see noticeable effects, such as the formation of HCN and changes in film morphology from triangular 111 crystals to square 100 crystals. Auger and SIMS analyses of the films showed that very little nitrogen was incorporated into the diamond films. Our observations are in agreement with a model for the surface chemistry of diamond growth in the presence of N2 by Goodwin & Butler [7].
(iv) Modelling
Modelling of the data obtained from the MBMS system is still being performed using the Chemkin suite of computer programs (Sandia Labs). Initial results were obtained for the data previously measured in the HF system [8,9]. This involved setting up the chemical databases and reaction schemes for the various hydrocarbon/H2 reactions, and finding accurate values for the activation energies and the temperature-dependent rate constants for all of the important possible reaction pathways. This being done, we are now in the process of applying these packages to the MBMS data obtained from the MW system.
Figures
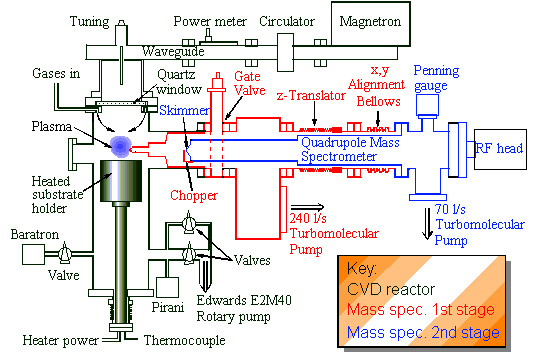
Fig.1. A schematic diagram of the MBMS-MW reactor
system.
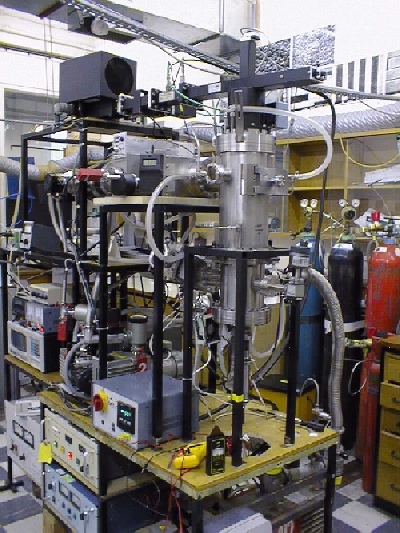
Fig.2. A photograph of the MW reactor and MBMS
assembly.

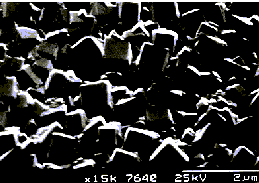
Fig.3. (left) Microcrystalline diamond film deposited using
the MW reactor using 1%CH4/H2 gas mixtures
and 1 kW.
Fig.4. (right) Microcrystalline diamond film deposited
using the MW reactor using 1%CH4/H2 with
1%N2 at 1 kW. The film morphology now shows a
preference for the {100} square facets, with an increased growth
rate.
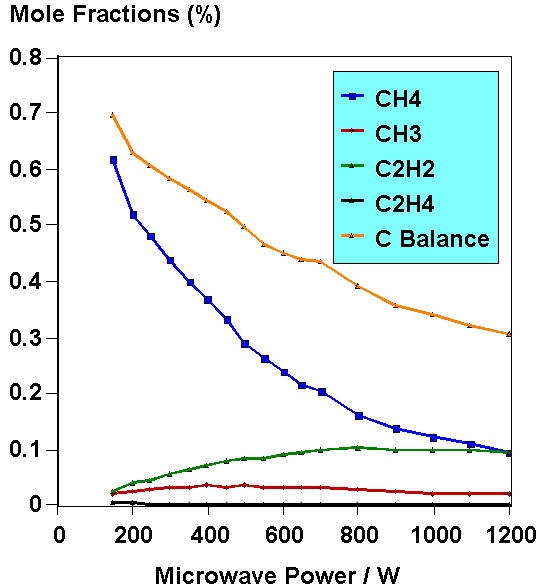
Fig.5. Example of hydrocarbon species mole fractions measured
by MBMS as a function of applied MW power. This was for 2% CH4
in H2. Taken from ref.[5].
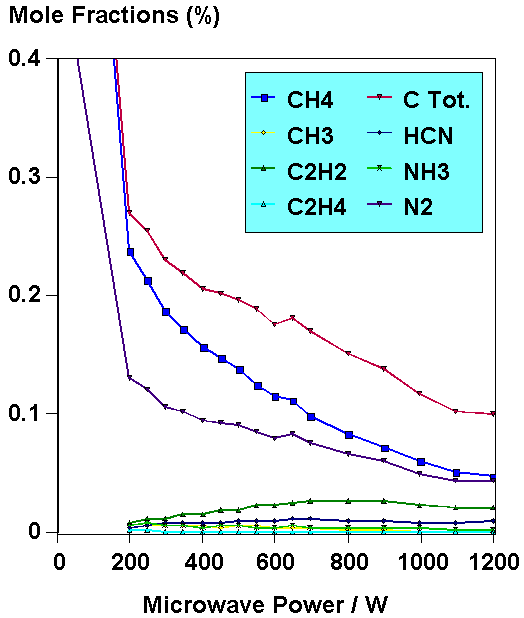
Fig.6. Example of hydrocarbon and nitrogen-containing species
mole fractions measured by MBMS as a function of applied MW power.
This was for 1% CH4 + 0.5% N2 in H2.
Taken from ref.[6].