Chapter 5 - Results for C/H/Cl Systems
5.1
Introduction
For hot filament and
microwave, rf and dc plasma CVD systems,3.4,5.1 deposition of high
quality diamond films using hydrogen/carbon gas mixtures at any appreciable
rates can be achieved only if the substrate temperature is maintained at around
900C. As a result, the choice of substrate is limited to very few
materials. Owing to its remarkable
chemical, physical and optical properties,5.2 diamond has great
potential for many industrial applications so, by altering the conditions which
produce high quality films at lower substrate temperatures, its applications
may extend to the less thermally stable materials such as aluminium, stainless
steel, glass, copper and plastics.
This
has prompted the study of CVD diamond growth using other precursor gas
mixtures. For instance, it is possible
to grow CVD diamond films using either oxygen containing source gases, e.g. CH3OH5.3
and CO,5.4 in excess H2 or by the addition of small
amounts of O25.5 to the standard hydrocarbon/H2
source gas mixture. The presence of gas
phase oxygen can enhance diamond growth rates and also enable diamond synthesis
at lower substrate temperatures. A
survey of the different C/H/O source gas mixtures used to produce diamond films
is summarised in the well known phase diagram of Bachmann et al.5.6 The
apparent catalytic effect of oxygen in the CVD process enabling diamond growth
at substrate temperatures lower than the standard (~900°C) may therefore have
possible important implications for coating such lower melting point materials
as aluminium, or materials that are unstable at high temperatures in the
reducing atmosphere typical of the CVD process. Such advantages cannot be fully exploited in hot filament CVD
(HFCVD) reactors, though, because the presence of oxygen rapidly degrades the
heated filament. In recent reports
however, lower-temperature diamond deposition has also been achieved using
either chlorine containing source gases, e.g. CH4-nCln (n = 1-4) diluted in hydrogen5.7-5.11
or by the addition of small amounts of Cl25.12 or HCl5.13
to the standard methane/hydrogen gas mixture.
One
study,5.7 using monosubstituted halocarbons CH3F, CH3Cl,
CH3Br or CH3I in H2 as the input gas mixture
in a hot filament reactor, showed that with CH3Cl the diamond growth
rates increased relative to those found using CH4 as the
precursor. It was concluded that the
increased reactivity of CH3Cl compared to the other methyl halides
stems from the difficulty, in the one case, of forming atomic fluorine from CH3F
and, at the other extreme, the inability of Br and I atoms to abstract
terminating hydrogen atoms from the growing diamond surface. Other studies using chloride source gases
(CH3Cl, CH2Cl2, CHCl3 and CCl4)
both in hot filament reactors5.8,5.9 and in microwave plasmas5.10,5.11
have also shown that chloromethanes produce a slightly higher diamond growth
rate than with methane at normal substrate temperatures (ca. 900C), and all indicate that
this difference is more pronounced at lower substrate temperatures. Recently,5.12 a study using
chlorine with typical H2/Cl2/CH4 ratios of
100/5/1 demonstrated that similar growth rates can be achieved at substrate
temperatures 150C lower than that found for
typical H2/hydrocarbon gas mixtures, and that the addition of a few
percent of HCl to the standard CH4/H2 mixture could cause
a tenfold enhancement in growth rates at 670°C.5.13
A
number of possible gas phase and gas-solid heterogeneous reaction mechanisms
have been suggested for the apparent catalytic activity of chlorine in the CVD
process. The C-Cl bond strengths in the
various chloromethanes are weaker than the C-H bond in methane (see Table 5.1)
so rupture of the C-Cl bond, by reaction with H atoms, can be achieved more
readily to produce active methyl or chloromethyl (CH3-nCln, n=1-3) radicals. The latter species have been suggested as
being more effective growth precursors than methyl5.7 because of the
possibility of facile surface dehydrochlorination reactions.5.8 Alternatively, the catalytic properties of
chlorine in the CVD process may be due to reactions with terminal C-H at the
diamond surface, or the possibility of forming surface bonded C-Cl. The terminating H and Cl atoms essentially
serve the same purpose in suppressing formation of sp2 bonding at
the growing surface at high substrate temperatures. Abstraction of surface Cl atoms by atomic H, or removal of
surface adsorbed H by atomic chlorine, has a significantly lower activation
energy than H abstraction of surface H thereby enabling active surface sites to
be created at lower substrate temperatures.5.13
Bond Bond strength (kJ/mol)
H-H 432
Cl-Cl 239
H-Cl 428
H-F 564
H-CH3 435
Cl-CCl3 305
Cl-CHCl2 331
Cl-CH2Cl 346*
Cl-CH3 352
F-CF3 540
Table 5.1. Bond dissociation energies
( taken from Reference 5.14 except for * which is taken from
Reference 5.15).
The aim of the present work
is to characterise the gas phase environment, and its variation with filament
temperature, when chlorine containing precursors CH4-nCln (n=1-4) in H2 and CH4/Cl2/H2
mixtures are inputted as the source gases.
Such information provides insight into the gas-phase chemical kinetics
prevalent in the CVD process. We also
wish to understand this behaviour in terms of changes in the gas-phase
chemistry and/or the different gas-solid heterogeneous reactions when chlorine
is present in the gas mixture.
5.2
Experimental Details
(a) Deposition experiments
Table 5.2 below shows the growth conditions used for the C/H/Cl system. Full experimental details on deposition techniques are given in Chapter 3 (Sections 3.1 and 3.2).
Pressure 20 Torr
Gases (1) CH4-nCln (n = 1-4),
(2) 1% CH4 in Cl2/H2, the amount of chlorine
varying from 1-4%.
Total gas flow rate 100 sccm
Substrate type Si (100) substrates
(manually abraded)
Substrate temperature ~ 900°C
Filament temperature 2300-2400°C (filament current 6![]() -6
-6![]() A)
A)
Filament/substrate distance 4 mm
Deposition time 6 hours
Table 5.2. Deposition conditions used
for C/H/Cl system.
(b) Film analysis
The as-grown diamond films were investigated by scanning electron microscopy (SEM) and Auger electron spectroscopy (AES). The AES analysis was carried out using a Perkin-Elmer PHI 595 scanning Auger microprobe.
(c) Gas phase composition measurements
Gas-phase product distributions were monitored as a function of the filament temperature using the differentially pumped molecular beam mass spectrometer (MBMS). Filament temperatures were measured using a two-color optical pyrometer (Land Infrared) and the filament-to-sampling orifice distance was held at 4 mm for all readings. The absolute concentrations of the species monitored are determined by direct room temperature calibration with mixtures of known composition. Full description of the molecular beam mass spectrometer and the calibration procedures are given in Chapter 3 (Sections 3.3 to 3.5).
A series of quantitative
measurements of the stable gas-phase species and CH3 radicals was
made using, respectively, chloromethane (CH3Cl), dichloromethane (CH2Cl2),
trichloromethane (CHCl3) or tetrachloromethane (CCl4) in
H2 (See Figures 5.4 (a) to 5.4 (d) ). The signal intensities of the species monitored have been
converted into mole fractions using the step-by-step procedure described in
Chapter 3 (See Section 3.6). The
following results are therefore presented as species concentrations as a
function of filament temperature with no correction being made as a result of
thermal diffusion (experimental data sheets for each precursor gas can be found
in the appendix that follows this chapter).
Detection of all stable
carbon and chlorine containing species, other than C2H4,
was made with an ionizing electron energy of 15.6 eV. An electron energy of 13.6 eV was used for C2H4
in order to minimize interference from unwanted ions with the same
mass-to-charge ratio or those arising from fragmentation of other ionic species
(See Section 3.5 (b) ). For the same
reasons, all CH3+ signal measurements were made using an
ioniser voltage of 13.6 eV.
However corrections have been made to the CH3+ signal
arising from the dissociative ionization of CH3Cl (See Section 3.6
(b) ).
As previously mentioned (See
Section 3.5 (c) ), introduction of a heavy species, such as Cl2, in
the gas mixture results in mass discrimination effects in the molecular beam,3.11
particularly when the concentration is greater than ~1%. Consequently, the lighter species, such as
CH4, are preferentially excluded from the centre of the beam by the
heavier Cl2 molecules (See Figure 3.20). When performing gas-phase composition measurements using CH4/Cl2/H2
containing gas mixtures, a minor modification had to be made to the direct room
temperature calibration procedure in order to determine accurate concentrations
for each species monitored. This was
achieved by measuring the signal loss for all the species monitored during the
calibration process when Cl2 is added to the gas mixture. For example, if 1% Cl2 was
introduced to the standard 1% CH4/H2 during sampling,
then the same concentration of Cl2 was added to that gas mixture
when calibrating for methane. The
attenuated CH4+ signal therefore constitutes 1% in the
gas-phase when 1% Cl2 is present in the process gas mixture,
allowing for the mass discrimination effects.
Similar corrections were made when 2% Cl2 and 4% Cl2
were added to the CH4/H2 gas mixture during MBMS
measurements.
5.3 Analysis
of the diamond films
Scanning
electron micrographs of diamond films grown on silicon when using 1% each of CH4-nCln (n=1-4) in H2
as the process gas mixture are shown in Figure 5.1. The SEM images in Figure 5.2 show that polycrystalline diamond
films were also grown successfully using up to 3 % Cl2 in a 1% CH4/H2
gas mixture. Further addition of
chlorine (~4%) leads to a significant change in the film morphology (See Figure
5.2(d) ) with a concomitant reduction in the film quality and growth rates.
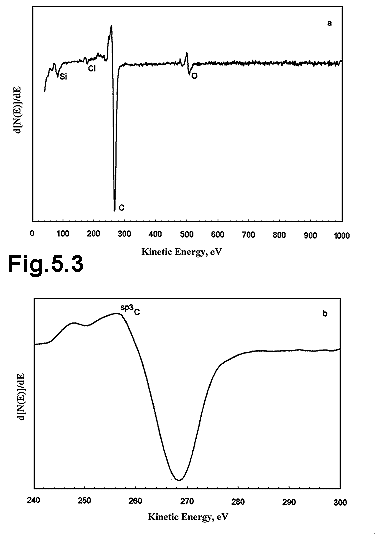
Figure
5.3 (a) shows a typical AES spectrum of the diamond film surface grown using a
2% Cl2 was introduced in a 1% CH4 in H2 gas
mixture. The vertical scale on the AES
spectrum is a differential scale used in order to display the peaks more
clearly. The fine structure of the KLL carbon peak (Figure 5.3 (b) ) is
characteristic of that found with natural diamond.5.16, 5.17 The peak is shifted slightly to lower
energies (by ca. 4 eV) compared with
the peak obtained from the very poor quality diamond when 4% Cl2 was
used for growth. This energy shift
arises mainly from charging effects on the diamond relative to the more
conducting amorphous or graphitic carbon.
Also evident on the spectrum is a small peak at an electron energy of 180 eV indicative of the presence of chlorine on the diamond surface. After argon ion sputtering for 30 seconds, resulting in etching of the diamond film to a depth of ~120 Å, the chlorine was no longer detectable indicating that this chlorine is present only on the film surface and not in the bulk diamond. In AES, the concentration of any particular element can be calculated from its peak intensity multiplied by a sensitivity factor. The sensitivity factors are determined empirically and are unique to each element. The relative intensity of the surface Cl peak suggests a chlorine concentration of ~0.5%; however, this is an average value obtained over the top four diamond monolayers. Given that the chlorine is present only on the diamond surface then about 2% of the surface carbon atoms are terminated by chlorine rather than hydrogen.
Similar
AES results indicating high quality diamond with only a trace of chlorine on
the film surface were obtained for all the as-grown films where the Cl atom
input fraction was 0.06 or below (i.e.
£3% Cl2
in the process gas mixture). However,
for growth conditions where the Cl fraction was greater than 0.06 the fine
structure of the KLL carbon peak
showed that the resulting films were of poor quality with significant
deposition of non-diamond carbon phases.
Also incorporated into the film were appreciable amounts of tantalum and
chlorine, indicating degradation of the filament at higher chlorine
concentrations.
a)  |
b)  |
c) 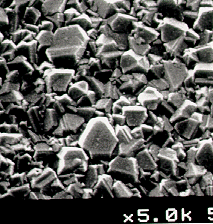 |
d)  |
Figure 5.1. Scanning
electron micrographs (SEM’s) of diamond films grown on silicon using input gas
mixtures of 1% each of: (a) CH3Cl; (b) CH2Cl2;
(c) CHCl3; and (d) CCl4 in hydrogen.
a)  |
b)  |
c) 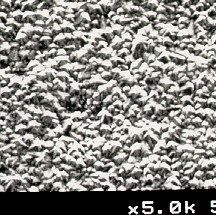 |
d)  |
Figure 5.2. Scanning
electron micrographs (SEM’s) of diamond films grown on silicon using input gas
mixtures of 1% CH4 in H2 with additional Cl2:
(a) 1%; (b) 2%; (c) 3%; and (d) 4%.
5.4 Gas
composition versus filament temperature for a variety of chlorine containing
precursor gases in H2
(a) Chloromethane (1% CH3Cl
in H2)
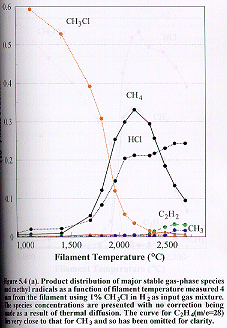
The
effects of the variation of filament temperature between room temperature (25°C) and 2690°C on the mole fractions of
the major dissociation products of chloromethane gases [CH4 (m/e =16), C2H2 (m/e =26), C2H4 (m/e =28) and HCl (m/e =36 for H35Cl)] were examined for an initial
feedstock of 1% CH3Cl in H2 measured 4 mm from the filament at 20 Torr (Figure 5.4 (a)
). Inspection of Figure 5.4 (a) shows
that there is a rapid decrease in the concentration of CH3Cl for
filament temperatures above ~1700°C; at the same
time there is significant rise in the CH4 and HCl mole fractions
such that these become the dominant species at ~2100°C. However, at filament temperatures above ~2100°C the CH4
concentration decreases steadily, and a subsequent increase in the C2H2
and CH3 concentrations is observed.
(b) Dichloromethane (1% CH2Cl2
in H2)
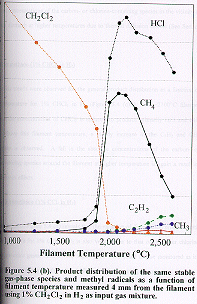
Figure 5.4 (b) shows how the
distribution of the same major product gases vary as a function of filament
temperature for an initial CH2Cl2/H2 feedstock
ratio of 1% measured at 4 mm from the filament. The gas phase composition as a function of filament temperature
for dichloromethane are remarkably similar to that for chloromethane. The concentration of CH2Cl2
decreases rapidly for filament temperatures above ~1700°C, and a corresponding rise
in the HCl concentration is observed reaching a maximum value at ~2100°C. Similarly, at this filament temperature the dominant hydrocarbon
species is methane, while at higher temperatures acetylene (and CH3
radicals) are formed. Note that the
absolute concentrations of the carbon- or chlorine-containing species in the
vicinity of the filament diminish at higher temperatures due to thermal
diffusion effects (See Section 3.5 (e) ).
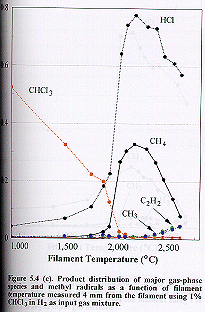
(c) Trichloromethane (1% CHCl3
in H2)
Similar
trends were observed for the general product distribution as a function of
filament temperature for 1% CHCl3 in H2 (Figure 5.4 (c)
). At ~2100°C filament temperature the
concentration of CHCl3 is reduced to produce primarily HCl and CH4
species. Above this filament
temperature, a steady increase in the C2H2 and CH3
concentrations is observed. A fall in
the absolute concentrations of the carbon- or chlorine-containing species
around the filament at higher temperature is again a result of thermal
diffusion effects.
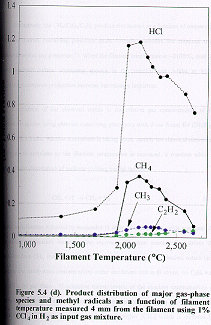
(d) Tetrachloromethane (1% CCl4
in H2)
Inspection
of Figure 5.4 (d) reveals that the variation of dissociation products with
filament temperature for 1% CCl4 in H2 is also very
similar to that of the other chlorine containing precursor gases. The C35Cl4+
signal (m/e=152) could not be monitored as its mass-to-charge ratio is beyond
the measurable range of the mass spectrometer.
Mechanism
Qualitatively, the CH4/C2H4/C2H2
product distribution as a function of temperature for all the chlorine
containing precursor gases strongly resembles that obtained when methane is
used as the precursor.3.1
When the filament temperature is ~2100C, methane is the dominant
carbon containing species in the sampled gas, whilst at higher temperatures
acetylene production becomes increasingly important.
Comparison of the observed
trends in the relative gas concentrations of the hydrocarbon species using
chlorine containing precursors with those found for CH4/H23.1
suggests that a similar reaction mechanism is taking place, namely the chemical
conversion of methane to acetylene as the filament temperature is increased, a
reaction which is initiated by the dissociation of methane yielding methyl
radicals:
CH4 +
H ![]() CH3 + H2 (4.1)
CH3 + H2 (4.1)
Methyl recombination followed by successive H
abstraction reactions yields acetylene. As the filament temperature increases,
the increasing H atom concentration drives the equilibrium from CH4,
through C2H6 and C2H4 to C2H2. Due to its transient nature (and thus very
low steady-state concentration) under conditions rich in H atoms, no C2H6
was detected in the gas phase.
Quantitative measurements of the absolute concentrations of methyl
radicals were made simultaneously and are displayed in Figures 5.4 (a) to 5.4
(d). An increase in the filament
temperature results in higher [CH3] which is mirrored by increased
[C2H2]. As with
methane and other hydrocarbon precursor gases3.1, it is clear that
the CH3 radical is also an essential intermediate in the formation
of acetylene when chlorine containing input precursors are used.
For
example, inspection of Figure 5.4 (b) shows that the concentration of
dichloromethane diminishes rapidly for filament temperatures above ~1700C as the relatively weak
C-Cl bonds are broken in preference to C-H bonds by Cl abstraction with H
atoms, e.g.
CH2Cl2
+ H == CH2Cl + HCl
CH2Cl
+ H == CH2 + HCl, etc. (5.1)
This may reflect a role for direct pyrolysis
of, for example,
CCl4
® Cl + CCl3
(5.2)
followed by the equilibrium
Cl
+ H2 == H + HCl (5.3)
The key point here would be that pyrolytic H-H bond fission (in the gas phase or on the filament surface) would not be necessary to initiate the whole reaction sequence.
A corresponding rise in the
HCl concentration is observed reaching a maximum value at the same temperature
as methane (~2100C). At this temperature almost all of the
dichloromethane has been reduced.
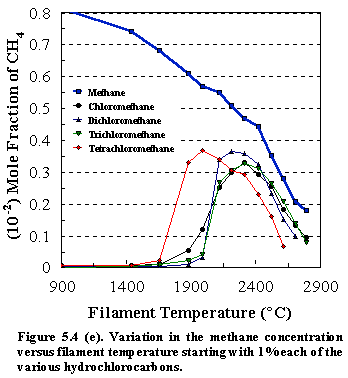
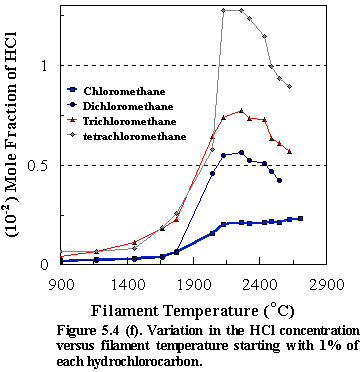
Similar trends were observed for all of the other CH4-nCln, (n=1-4) species, resulting in the
reduction of the precursors to produce primarily methane and HCl, (see Figs.
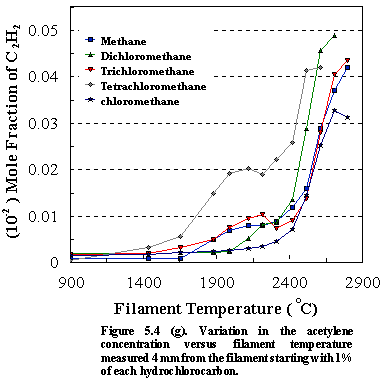
5.4 (e) and 5.4 (f) ). Figure 5.4 (g)
shows how the acetylene concentrations vary as a function of filament
temperature for input gas mixtures containing 1% CH4-nCln, (n=0-4) in H2. For all the
precursors, the C2H2 concentration rises sharply above
~2100C as the H atom
concentration increases. However, there is a significant increase in the C2H2
concentration for CCl4 and, to a lesser extent, for CHCl3
at lower temperatures (~1900C-2100C). As an alternative, we might envisage the
case where, for CHCl3 for example, a single C-Cl bond is cleaved to
form the CHCl2 radical which could then combine with a CH3
radical or react with CH4 to produce CHCl2CH3
(+ H in the latter case). Subsequent
elimination of two HCl molecules, reactions which are known to have reasonably
low activation barriers5.18, would yield acetylene. We should point out, however, that
radical-radical encounters are likely to be relatively infrequent in the gas
phase environment, nor have we been able to detect this intermediate, possibly
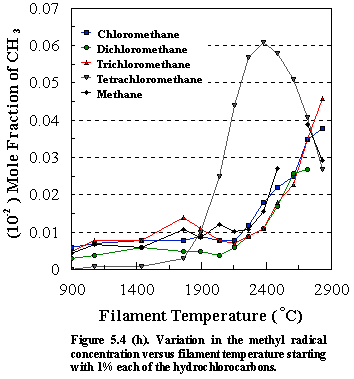
due to its instability in the presence of atomic hydrogen. However, close inspection of Figure 5.4 (h),
which shows how the methyl radical concentrations vary as a function of
filament temperature for different chlorocarbon input gas mixtures, indicates
that production of appreciable amounts of acetylene at lower filament
temperatures for the CCl4/H2 gas mixture may be due to
the presence of high concentrations of the methyl radicals in the gas
phase. In this case there is a greater
steady-state [CH3] and the increased likelihood of methyl
recombination, followed by consecutive H abstractions yields larger [C2H2]. Since the formation of [C2H2]
depends on k[CH3]2, the detection of large amounts of
gas-phase acetylene in a hot filament CVD reactor is generally taken as an
indicator of steady state [CH3] and thus of fast diamond film
growth.
5.5 Gas
composition versus filament temperature for various CH4/Cl2/H2
containing gas mixtures
Similar
measurements of the stable gas species were made using different CH4/Cl2/H2
gas mixtures subject to the same process conditions as the chlorine containing
hydrocarbon experiments.
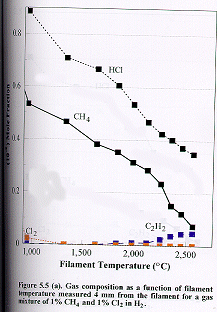
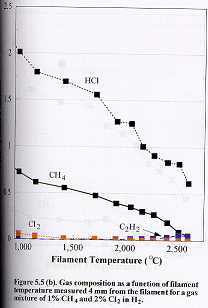
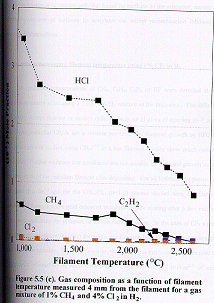
Figure
5.5 shows how the distribution of the major stable product gases [CH4,
C2H2, HCl and Cl2 (m/e =70 for 35Cl2)]
change as a function of filament temperature for (a) 1%, (b) 2% and (c) 4% Cl2
in the CH4/Cl2 gas mixtures in H2 measured 4
mm from the filament. Almost all the
molecular chlorine is dissociated when the filament temperature is ~1000C. The Cl atoms then undergo rapid reaction with hydrogen to form
HCl.
Cl
+ H2 == H + HCl (5.3)
The absolute measured concentrations of both
HCl and methane decrease with temperature
due to the thermal diffusion effects mentioned previously. At temperatures of ~2100C and above there is a
striking similarity in the measured C/Cl product distribution with that
obtained with 1% CH2Cl2 in H2 as the input gas
mixture (c.f. Figure. 5.4 (b) ). In these two cases the relative C/Cl input
mole fractions in H2 are identical.
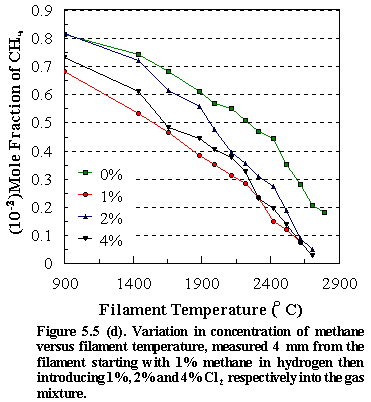
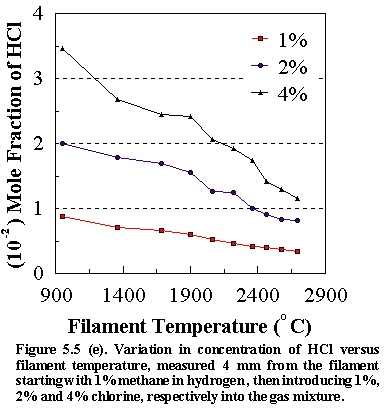
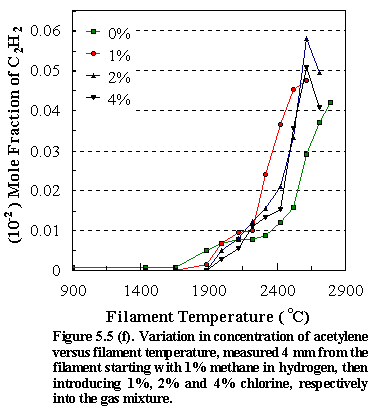
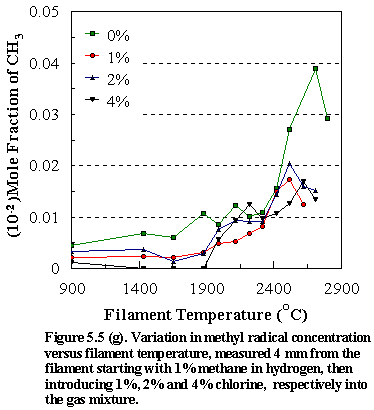
Figure
5.5 shows the temperature variation of: (d) [CH4], (e) [C2H2],
(f) [HCl] and (g) [CH3] for different input concentrations of Cl2
(0%, 1%, 2%, 4%) in the CH4/Cl2 gas mixtures in H2. As with the chlorine containing hydrocarbons
the observed trends for the hydrocarbon species at and above filament
temperatures ~2100C indicate that the reaction mechanism is congruent with that found
for methane as the precursor, namely the chemical conversion of methane to
acetylene via methyl recombination followed by successive H abstractions.
5.6 Gas
composition versus filament temperature using 1% CF4 in H2
No
significant concentrations of CH4, C2H4, C2H2
or HF were detected at any filament temperature when using a 1:100 CF4/H2
mixture as the precursor. The
difficulty in producing atomic fluorine or methyl radicals, or in an H atom
abstracting an F atom from CF4 suggests that CF4 is not a
suitable precursor for diamond growth by HFCVD and, indeed, growth studies using
CH3F5.7 in a hot filament reactor show much reduced
growth rates. Further evidence that
conditions are not suitable for diamond growth comes from the analysis of the
tantalum filament after deposition. For
all hydrocarbon precursors investigated thus far3.5 and for the CH4-nCln, (n=1-4)
species studied here, the filament carburises and forms a stable gold coloured
tantalum carbide coating after a few minutes of deposition, indicating the
presence of carbon radical species. In
the case of CF4 a grey film (presumably tantalum fluoride) forms on
the filament and the filament temperature tends to be rather unstable. We note, however, that C/H/F gas mixtures
have been used to grow diamond successfully by microwave plasma CVD, and MBMS
studies5.19 of such systems indicate that HF is the major sink for
fluorine.
5.7
Discussion
As
with the hydrocarbon precursor gases,5.4 chlorine containing
hydrocarbons are reduced to methane at very similar temperatures, the other
major product being hydrogen chloride which is formed in near stoichiometric
amounts. At higher filament
temperatures the temperature variation of the relative CH4/C2H4/C2H2
product distribution generally mirrors that found for the hydrocarbon
precursors at similar C/H2 input ratios. Only with CHCl3 or CCl4 do we observe
significant acetylene formation at considerably lower filament temperatures
which may indicate the presence of transient chlorine-containing radical
species supplementing the CH3 radicals as precursors to acetylene
production. For the CCl4/H2
gas mixture however, formation of appreciable amounts of acetylene at lower
filament temperatures may be a consequence of high concentrations of the methyl
radicals present in the gas phase.
Comparison
of Figures 5.6 (a) and 5.6 (b) indicates that at these filament temperatures
(2300°C) both the
chlorocarbon and Cl2 input precursors are completely dissociated
producing HCl in near stoichiometric proportions. Thermal diffusion effects again account for the reduction in the
concentration of HCl measured 4 mm from the filament. At such filament temperatures the H atom
concentration is of the order of 1-2%5.20 and abstraction of Cl from
the relatively weak Cl-Cl and C-Cl bonds (see Table 5.1) by H atoms to form HCl
is predicted on thermodynamic grounds.
Indeed, reduction of the chloromethanes to produce HCl is observed to be
largely complete at filament temperatures of ~1800°C and that of Cl2
at temperatures less than 900°C.5.21
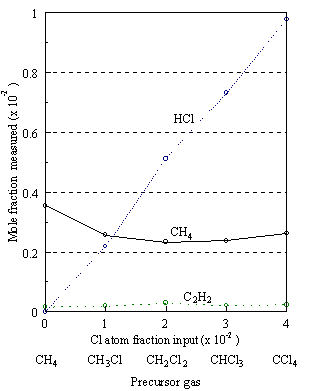
Figure 5.6 (a). Gas composition versus Cl atom fraction input in the feed gas using 1% each of CH4-nCln (n=0-4) in H2 at 2300C, measured 4 mm from the filament.
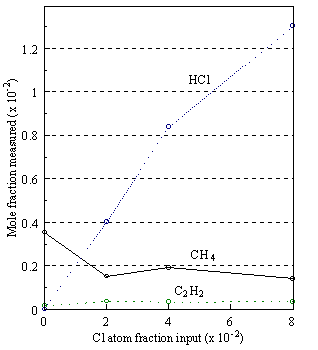
Figure 5.6 (b). Gas composition versus Cl atom fraction input in the feed gas using 1% CH4 in H2 with additional Cl2 varying from 0-4% at 2300C, measured 4 mm from the filament.
The breakdown of the
chlorocarbon precursors to form HCl produces a concomitant increase in the
concentration of methane such that at 2300°C it is the dominant
carbon-containing species. The H atom
concentration at these temperatures may also initiate H abstraction from
methane yielding significant amounts of methyl radicals. Methyl recombination to form C2H6
followed by successive H abstractions produces significant amounts of (C2H2),
the other main carbon containing species observed (see Figure 5.6). No C2H6 and only small
concentrations of C2H4 were detected, presumably because
their thermodynamic instability in the presence of atomic hydrogen leads to low
steady-state concentrations. Previous
gas-phase composition studies on CH4/H2 mixtures3.1
indicate that C2H2 is the most stable two-carbon species
as the H atom concentration increases, and at standard growth temperatures a CH4
to C2H2 ratio of ~8:1 is observed.
Previous
reports5.7,5.8,5.13 have speculated upon the relative importance of:
(a) modification of the gas-phase chemistry enhancing the production of diamond
precursor species, or (b) fast reaction of atomic hydrogen with surface bonded
C-Cl and atomic Cl with surface bonded C-H which open up active surface growth
sites at reduced substrate temperatures, as possible explanations for the
observations that addition of small amounts of chlorine in the CVD process
encourages diamond growth at lower substrate temperatures.
The
inability to detect the CH2Cl radical in the present MBMS study
suggests that its concentration must be at least an order of magnitude smaller
than that of CH3 at filament temperatures which produce optimum
diamond growth (2200-2400C). This is presumably because the residual CH4
concentration at these elevated temperatures is several times higher than that
of CH3Cl even when CH3Cl is the input precursor; for
dichloromethane and trichloromethane no significant concentration of CH3Cl
is measurable. This is to be expected
since thermodynamic and kinetic arguments suggest that the C-Cl bond is likely
to be broken in preference to the C-H bond.
Therefore, we do not believe that gas-phase chemistry involving
chlorohydrocarbon radicals can be primarily responsible for enhanced growth
rates at lower substrate temperatures.
However, it is possible that the addition of the CH2Cl
radical to the diamond surface and the subsequent elimination of HCl may occur
more readily than addition of the CH3 radical followed by
elimination or successive abstraction of hydrogen at lower substrate
temperatures.
At filament temperatures
above 2000C most of the chlorine is
rapidly abstracted by H atoms from the parent chlorohydrocarbon to produce HCl
in amounts proportional to the Cl mole fraction in the source gas. Similarly Cl2 is almost entirely
reduced to HCl at filament temperatures above 1000°C. However, in this case it is atomic chlorine produced by the
thermal dissociation of Cl2 which reacts rapidly with molecular
hydrogen to form HCl. It has been
proposed that the ability of chlorine to play a part in CVD diamond deposition
stems from the interchangeability of atomic Cl with atomic H in an HCl/H2
atmosphere. Calculations by Bai et al.5.7
indicate that for the reaction,
kf
H + HCl ===
Cl + H2 (5.3)
kb
the equilibrium constant (Keq) is close to unity over a
large temperature range and the forward and backward rate constants (kf and kb) are large (See Table 5.3), thereby allowing rapid
equilibration at typical substrate temperatures. Therefore, the relative concentration ratio of Cl/H atoms is
proportional to the Cl fraction in the source gas mixture regardless of the
form of chlorine in the input mixture.
|
|
log kf |
log Keq |
|
Reaction |
T= 500 K 800 K 1200 K |
T= 500 K 800 K 1200 K |
|
H + HF H2 + F |
-0.7 5.2 8.4 |
-13.9 -8.6 -5.7 |
|
H + HCl H2 + Cl H + CH4 ® CH3 + H2 H + CH3Cl ® CH3 + HCl H + CH3Cl ® CH2Cl + HCl Cl + CH4 ® CH3 + HCl Cl + CH3Cl ® CH2Cl + HCl |
11.7 12.2 12.5 8.9 11.0 12.1 9.6 11.0 12.2
11.7 12.1 12.8 12.2 12.6 12.8 |
0.2 0.03 -0.1 1.0 1.2 1.4 9.9 6.8 5.0 3.2 2.6 2.2 0.8 1.2 1.4 3.0 2.6 0.0 |
Table 5.3. Equilibrium and forward rate constants vs. temperature (taken from Reference 5.7).
The MBMS results combined
with the AES analysis5.22 of the as-grown diamond films are
consistent with the premise that Cl atoms play some part in gas-surface
reactions involved in the production of active surface growth sites at reduced
substrate temperatures. Chemical
kinetics calculations5.13 have shown that the rate of H abstraction
by Cl atoms from a (110) diamond surface at 670°C is some 60 times faster
than abstraction by gas-phase H atoms.
As indicated above, the Cl atom concentration at standard filament
temperatures is directly related to the Cl fraction in the input gas,
regardless of the form of the chlorine precursor. Our chlorine-assisted diamond growth studies shows that if the Cl
input fraction is too high (>0.06) the quality
of the diamond grown at normal substrate temperatures (~900°C) is reduced, probably
because a large fraction of surface sites are activated, leading to the
graphitisation of the diamond surface.
These findings broadly agree with the diamond growth domain indicated in
the C/H/Cl ternary gas-phase composition diagram of Bachmann et al.5.23 However, at lower substrate temperatures the
presence of such chlorine concentrations in the process gas mixture would
result in an increased deposition rate due to the greater efficiency of surface
hydrogen abstraction by Cl atoms.
The
inability of fluorine containing precursors to participate in the hot filament
CVD of diamond results from the strength of the C-F bond. Our gas-phase composition findings when
using 1% CF4 in H2 indicate that very little CH4
or HF are produced; further, even if we could produce atomic fluorine, the
equilibrium
H
+ HF == H2 + F (7)
lies far to the
left in a hydrogen atmosphere (See Table 5.3).
5.8
References
5.1 M.N.R. Ashfold, P.W. May, C.A. Rego and
N.M. Everitt, Chem. Soc. Rev., 23, 21 (1994).
5.2 J.C. Angus, Annu. Rev. Mater. Sci., 21, 221 (1991).
5.3 Y. Hirose and Y. Terasawa, Jpn. J. Appl.
Phys., 25, L519 (1986).
5.4 Y. Saito, K. Sato, K. Gomi and H.
Miyadera, J. Mater. Sci., 25, 1246
(1990).
5.5 T. Kawato and K. Kondo, Jpn. J. Appl.
Phys., 26, 1429 (1986).
5.6 P.K. Bachmann, D. Leers and H. Lydtin,
Diamond and Relat. Mater., 1, 12
(1991).
5.7 B.J. Bai, C.J. Chu, D.E. Patterson, R.H.
Hague and J.L. Margrave, J. Mater. Res.,
8, 233 (1993).
5.8 F. C.-N. Hong, G.-T. Liang, J.-J. Wu, D.
Chang and J.-C. Hsieh, Appl. Phys. Lett., 63, 3149 (1993).
5.9 F. C.-N. Hong, J.-J. Wu, C.-T. Su and
S.-H. Yeh, in Advances in New Diamond Science and Technology, MYU,
Tokyo, p.85 (1994).
5.10 C.H. Chu and M.H. Hon, Diamond Relat.
Mater., 2, 311 (1993).
5.11 T. Nagano and N. Shibata, Jpn. J. Appl.
Phys., 32, 5067 (1993).
5.12 C. Pan, C.J. Chu, J.L. Margrave and R.H.
Hague, J. Electrochem. Soc., 141,
3246 (1994).
5.13 N.J. Komplin, B.J. Bai, C.J. Chu, J.L.
Margrave and R.H. Hague, in Diamond Materials
(The Electrochemical Society proceedings Series, Pennington, NJ, 1993) Vol.
93-17, p.385.
5.14 Tables
of Physical and Chemical Constants, edited by G.W.C. Kaye and T.H. Laby,
(Wiley, New York 1989).
5.15 Ref.
Data on Atoms, Molecules and Ions, edited by A.A. Radzig and B.M. Smirnov,
Vol. 31 (Springer, Berlin 1985).
5.16 P.G. Lurie and J.M. Wilson, Surface Sci., 65, 476 (1977).
5.17 Q.S. Chia, C.M. Younes, P.G. Partridge,
G.C. Allen, P.W. May and C.A. Rego, J. Mater. Sci., 29, 6397 (1994).
5.18 W. Ho, R.B. Barat and J.W. Bozzelli,
Combustion and Flame, 88, 265
(1992).
5.19 C. Fox and W.L. Hsu, private communication.
5.20 D.S. Dandy and M.E. Coltrin, J. Appl.
Phys., 32, 5067 (1993).
5.21 C.A. Rego, R.S. Tsang, P.W. May, M.N.R. Ashfold
and K.N. Rosser, J. Appl. Phys., 79,
7264 (1996).
5.22 R.S. Tsang, C.A. Rego, P.W. May, J. Thumim,
M.N.R. Ashfold, K.N. Rosser, C.M. Younes and M.J. Holt, Diamond Relat. Mater., 5, 359 (1996).
5.23 P.K. Bachmann, H.J. Hagemann, H. Lade, D.
Leers, F. Picht, D.U. Wiechert and H.
Wilson, Mater. Res. Soc. Symp. Proc., 339, 267 (1994).
5.9 Appendix:
(I) Ionization potentials (I.P.) of the various precursor gases used (taken from Reference 3.19)
|
Precursor Gas |
Chemical
Formula |
Ionization
Potential (eV) |
|
Methane |
CH4 |
12.98 |
|
Chloromethane |
CH3Cl |
11.28 |
|
Dichloromethane |
CH2Cl2 |
11.35 |
|
Trichloromethane |
CHCl3 |
11.42 |
|
Tetrachloromethane |
CCl4 |
11.47 |
|
Hydrogen Chloride |
HCl |
12.74 |
|
Chlorine |
Cl2 |
11.48 |
|
Tetrafluoromethane |
CF4 |
<15.00 |
(II) Experimental Data
The
following experimental values have been converted from raw MBMS data into
species mole fractions as a function of filament temperature for chlorine
containing precursors CH4-nCln (n=1-4) in H2 and CH4/Cl2/H2
gas mixtures. The species concentration
are presented with no correction being made as a result of thermal
diffusion. Typical uncertainties in
these values can be estimated by comparison with Figures 4.4 (a) to (d).
1% CH3Cl in H2 at 20
Torr vs. Filament Temperature
MS Probe
Parameters: -6% (DISCRIM), 0% (DELTAM), -40% (RES’N), 2650V
(SEM), 3.0V (CAGE), 120mA (EMISS).
MS Pressure =
8x10-7 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
CH3Cl (15.6eV) |
CH3 (13.6eV) |
|
25 |
0.008 |
0.002 |
0.003 |
0.000 |
1.000 |
0.000 |
|
900 |
0.007 |
0.002 |
0.003 |
0.010 |
0.740 |
0.006 |
|
1070 |
0.007 |
0.002 |
0.003 |
0.020 |
0.590 |
0.007 |
|
1400 |
0.010 |
0.002 |
0.002 |
0.022 |
0.530 |
0.008 |
|
1700 |
0.056 |
0.002 |
0.006 |
0.043 |
0.390 |
0.008 |
|
1820 |
0.122 |
0.003 |
0.005 |
0.083 |
0.310 |
0.009 |
|
1960 |
0.254 |
0.003 |
0.005 |
0.145 |
0.120 |
0.008 |
|
2060 |
0.299 |
0.003 |
0.003 |
0.204 |
0.060 |
0.008 |
|
2160 |
0.332 |
0.005 |
0.004 |
0.212 |
0.040 |
0.012 |
|
2270 |
0.295 |
0.007 |
0.006 |
0.211 |
0.020 |
0.018 |
|
2370 |
0.257 |
0.014 |
0.009 |
0.217 |
0.010 |
0.022 |
|
2480 |
0.185 |
0.025 |
0.012 |
0.230 |
0.004 |
0.025 |
|
2580 |
0.133 |
0.033 |
0.008 |
0.243 |
0.000 |
0.035 |
|
2690 |
0.095 |
0.031 |
0.007 |
0.243 |
0.000 |
0.038 |
1% CH2Cl2 in H2
at 20 Torr vs. Filament Temperature
MS Probe Parameters: -6% (DISCRIM), -20% (DELTAM), -40% (RES’N), 2600V (SEM), 3.0V (CAGE), 140mA (EMISS).
MS Pressure =
1.4x10-6 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
CH2Cl2 (15.6eV) |
CH3 (13.6eV) |
|
25 |
0.004 |
0.002 |
0.000 |
0.000 |
1.000 |
0.000 |
|
900 |
0.004 |
0.002 |
0.002 |
0.015 |
0.599 |
0.003 |
|
1250 |
0.004 |
0.002 |
0.002 |
0.024 |
0.495 |
0.004 |
|
1480 |
0.005 |
0.002 |
0.003 |
0.028 |
0.432 |
0.006 |
|
1750 |
0.012 |
0.002 |
0.001 |
0.035 |
0.330 |
0.005 |
|
1860 |
0.032 |
0.003 |
0.002 |
0.070 |
0.273 |
0.005 |
|
1980 |
0.341 |
0.005 |
0.002 |
0.457 |
0.028 |
0.004 |
|
2070 |
0.365 |
0.008 |
0.005 |
0.549 |
0.016 |
0.006 |
|
2140 |
0.361 |
0.009 |
0.006 |
0.564 |
0.009 |
0.009 |
|
2240 |
0.326 |
0.014 |
0.006 |
0.524 |
0.002 |
0.011 |
|
2380 |
0.233 |
0.029 |
0.010 |
0.511 |
0.002 |
0.017 |
|
2505 |
0.151 |
0.046 |
0.010 |
0.471 |
0.000 |
0.026 |
|
2615 |
0.098 |
0.049 |
0.007 |
0.422 |
0.000 |
0.027 |
1% CHCl3
in H2 at 20 Torr vs. Filament Temperature
MS Probe Parameters: -6% (DISCRIM), -20% (DELTAM), -40% (RES’N), 2600V (SEM), 3.0V (CAGE) 140mA (EMISS).
MS Pressure =
1.4x10-6 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
CHCl3 (15.6eV) |
CH3 (13.6eV) |
|
25 |
0.003 |
0.003 |
0.001 |
0.000 |
1.000 |
0.000 |
|
900 |
0.003 |
0.002 |
0.002 |
0.040 |
0.524 |
0.005 |
|
1470 |
0.004 |
0.002 |
0.002 |
0.069 |
0.325 |
0.008 |
|
1730 |
0.011 |
0.003 |
0.002 |
0.111 |
0.224 |
0.008 |
|
1850 |
0.024 |
0.005 |
0.003 |
0.183 |
0.199 |
0.014 |
|
1920 |
0.042 |
0.008 |
0.003 |
0.226 |
0.128 |
0.011 |
|
2010 |
0.268 |
0.009 |
0.006 |
0.643 |
0.024 |
0.008 |
|
2070 |
0.305 |
0.011 |
0.006 |
0.742 |
0.016 |
0.007 |
|
2150 |
0.329 |
0.007 |
0.003 |
0.777 |
0.000 |
0.009 |
|
2270 |
0.313 |
0.009 |
0.007 |
0.737 |
0.000 |
0.011 |
|
2360 |
0.266 |
0.014 |
0.005 |
0.731 |
0.000 |
0.018 |
|
2440 |
0.207 |
0.028 |
0.009 |
0.634 |
0.000 |
0.023 |
|
2535 |
0.140 |
0.040 |
0.008 |
0.610 |
0.000 |
0.035 |
|
2610 |
0.080 |
0.043 |
0.008 |
0.571 |
0.000 |
0.046 |
1% CCl4 in H2 at 20
Torr vs. Filament Temperature
MS Probe Parameters: -6% (DISCRIM), -20% (DELTAM), -40% (RES’N), 2600V (SEM), 3.0V (CAGE) 140mA (EMISS).
MS Pressure =
7x10-7 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
CH3 (13.6eV) |
|
25 |
0.004 |
0.001 |
0.001 |
0.063 |
0.007 |
|
1360 |
0.007 |
0.001 |
0.002 |
0.121 |
0.012 |
|
1698 |
0.009 |
0.003 |
0.002 |
0.167 |
0.014 |
|
1910 |
0.025 |
0.006 |
0.003 |
0.298 |
0.028 |
|
2010 |
0.332 |
0.015 |
0.006 |
1.162 |
0.043 |
|
2130 |
0.369 |
0.019 |
0.006 |
1.184 |
0.060 |
|
2200 |
0.341 |
0.020 |
0.003 |
1.093 |
0.063 |
|
2250 |
0.306 |
0.019 |
0.007 |
1.035 |
0.062 |
|
2328 |
0.294 |
0.022 |
0.005 |
0.973 |
0.059 |
|
2395 |
0.231 |
0.026 |
0.009 |
0.980 |
0.045 |
|
2580 |
0.162 |
0.041 |
0.008 |
0.871 |
0.036 |
|
2670 |
0.069 |
0.042 |
0.008 |
0.758 |
0.028 |
1%
CH4 & 1% Cl2 in H2 at 20 Torr vs. Filament
Temperature
MS Probe Parameters: -3% (DISCRIM), -20% (DELTAM), -40% (RES’N), 2600V (SEM), 3.0V (CAGE) 140mA (EMISS).
MS Pressure =
8x10-7 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
Cl2 (15.6eV) |
CH3 (13.6eV) |
|
23 |
1.000 |
0.001 |
0.000 |
0.000 |
1.000 |
0.000 |
|
800 |
0.683 |
0.000 |
0.001 |
0.973 |
0.131 |
0.002 |
|
950 |
0.533 |
0.000 |
0.001 |
0.886 |
0.023 |
0.003 |
|
1330 |
0.467 |
0.000 |
0.003 |
0.710 |
0.000 |
0.002 |
|
1630 |
0.383 |
0.002 |
0.004 |
0.669 |
0.000 |
0.003 |
|
1830 |
0.354 |
0.007 |
0.002 |
0.607 |
0.000 |
0.005 |
|
1980 |
0.315 |
0.010 |
0.005 |
0.535 |
0.000 |
0.005 |
|
2120 |
0.286 |
0.010 |
0.004 |
0.469 |
0.000 |
0.007 |
|
2250 |
0.235 |
0.024 |
0.002 |
0.425 |
0.000 |
0.008 |
|
2350 |
0.152 |
0.037 |
0.002 |
0.403 |
0.000 |
0.015 |
|
2450 |
0.122 |
0.045 |
0.004 |
0.372 |
0.000 |
0.017 |
|
2560 |
0.075 |
0.048 |
0.004 |
0.349 |
0.000 |
0.013 |
1%
CH4 & 2% Cl2 in H2 at 20 Torr vs. Filament
Temperature
MS Probe Parameters: -3% (DISCRIM), -20% (DELTAM), -40% (RES’N), 2600V (SEM), 3.0V (CAGE) 140mA (EMISS).
MS Pressure =
8x10-7 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
Cl2 (15.6eV) |
CH3 (13.6eV) |
|
23 |
1.000 |
0.001 |
0.000 |
0.000 |
2.000 |
0.000 |
|
800 |
0.818 |
0.000 |
0.000 |
2.191 |
0.148 |
0.003 |
|
950 |
0.723 |
0.000 |
0.000 |
2.011 |
0.059 |
0.004 |
|
1130 |
0.614 |
0.000 |
0.000 |
1.798 |
0.036 |
0.001 |
|
1410 |
0.557 |
0.000 |
0.001 |
1.699 |
0.012 |
0.003 |
|
1710 |
0.476 |
0.005 |
0.002 |
1.560 |
0.013 |
0.008 |
|
1910 |
0.394 |
0.008 |
0.003 |
1.273 |
0.000 |
0.009 |
|
2050 |
0.355 |
0.012 |
0.002 |
1.251 |
0.000 |
0.009 |
|
2160 |
0.308 |
0.016 |
0.001 |
1.007 |
0.000 |
0.009 |
|
2270 |
0.275 |
0.021 |
0.003 |
0.911 |
0.000 |
0.014 |
|
2400 |
0.191 |
0.033 |
0.003 |
0.837 |
0.000 |
0.020 |
|
2510 |
0.091 |
0.060 |
0.001 |
0.822 |
0.000 |
0.016 |
|
2600 |
0.053 |
0.050 |
0.001 |
0.616 |
0.000 |
0.015 |
1%
CH4 & 4% Cl2 in H2 at 20 Torr vs. Filament
Temperature
MS Probe Parameters: -3% (DISCRIM), -20% (DELTAM), -40% (RES’N), 2600V (SEM), 3.0V (CAGE) 140mA (EMISS).
MS Pressure =
6.5x10-7 Torr
Species Mole
Fractions vs. Filament Temperature
|
Fil. Temp (°C) |
CH4 (15.6eV) |
C2H2 (15.6eV) |
C2H4 (13.6eV) |
HCl (15.6eV) |
Cl2 (15.6eV) |
CH3 (13.6eV) |
|
23 |
1.000 |
0.000 |
0.000 |
0.000 |
4.000 |
0.000 |
|
800 |
0.733 |
0.000 |
0.000 |
3.863 |
0.203 |
0.001 |
|
970 |
0.611 |
0.000 |
0.000 |
3.468 |
0.052 |
0.000 |
|
1150 |
0.485 |
0.000 |
0.000 |
2.683 |
0.027 |
0.000 |
|
1430 |
0.446 |
0.000 |
0.000 |
2.459 |
0.009 |
0.000 |
|
1700 |
0.407 |
0.003 |
0.002 |
2.427 |
0.000 |
0.006 |
|
1860 |
0.473 |
0.006 |
0.002 |
2.063 |
0.000 |
0.009 |
|
2010 |
0.327 |
0.011 |
0.001 |
1.935 |
0.000 |
0.013 |
|
2130 |
0.231 |
0.013 |
0.000 |
1.745 |
0.000 |
0.010 |
|
2250 |
0.198 |
0.015 |
0.001 |
1.427 |
0.000 |
0.011 |
|
2350 |
0.141 |
0.036 |
0.002 |
1.301 |
0.000 |
0.013 |
|
2470 |
0.075 |
0.053 |
0.004 |
1.163 |
0.000 |
0.017 |
|
2600 |
0.030 |
0.041 |
0.006 |
0.828 |
0.000 |
0.014 |