
Martin Hollamby - New Applications in CO2 Dispersions
PhD, October 2005 -


Martin carried out his undergraduate degree in this department, working for Paul Bartlett in his final year. His project mainly involves the synthesis of nanoparticles in condensed CO2 and is sponsored by Kodak.
The environmental threat posed by waste industrial solvent is well documented. In 2003 it was estimated that over 350,000 tonnes of volatile organic compounds, 34% of the total amount were generated from solvent usage. This problem has motivated the scientific world to research possible alternative "greener" solvents. One alternative is supercritical CO2 being cheap, tuneable, non-toxic, inert, non-flammable, recyclable and environmentally benign. Unfortunately CO2 alone is a poor solvent for most chemicals; surface active species are required to boost solubility [1], thus it is not usually possible to directly substitute it for conventional solvents. Suitable industrial applications must be found.
One such application could be a catalytic system, consisting of a surfactant stabilised nanoparticle solution. The possibilities extending from such a system are wide; it would offer 100% catalyst recovery (by merely lowering CO2 pressure), plus the solvent properties could also be tailored for higher efficiency by careful adjustment of temperature and pressure. The catalytic material employed in such a system could be synthesised in microemulsions, a technique which yields surfactant coated nanoparticles [2].
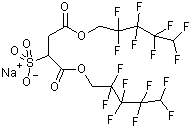
The choice of surfactant however is less obvious. Current technology presents no clear hydrocarbon surfactant candidate to stabilise the particles in CO2, although ongoing work in the Eastoe group looks promising [3]. Thus in this project we have turned back to fluorinated surfactants, in particular fluorinated AOT analogues DiHCF4 and DiCF4, which have been found to stabilise water-in-CO2 [3] and water-in-fluorocarbon microemulsions [4] at high pressure. We hope to find firstly a stable "fluorinated microemulsion" (water/f-surfactant/f-carbon) at ambient temperature, pressure. After this stage we will attempt to employ this microemulsion to synthesise nanoparticles and attempt to re-disperse them in CO2.
 |
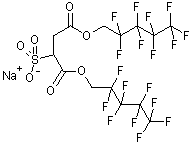 |
| DiHCF4 | DiCF4 |
[1] J Eastoe, S Gold Phys. Chem. Chem. Phys. 7 (2005) 1352
[2] Review paper - Recent Advances in Nanoparticle Synthesis in Reversed Micelles - yet to be published
[3] J Eastoe et al, Phys. Chem. Chem. Phys. 2 (2000) 5235
[4] Steytler et al, Langmuir, 19 (2003) 8715