Ammonia
This web site
aims to provide a full and comprehensive insight into ammonia production and
its history. Covered in this site are the following:
-
A brief insight into the chemistry
of ammonia. -
So how did the Haber-Bosch process
come about? -
So what is the Haber-Bosch
process? -
Why is ammonia so important? -
Other useful information.
A brief insight into the chemistry of ammonia:
Ammonia has a
triangular pyramidal geometry, and boiling points of 77.7*C and 33.5*C. In its
pure form ammonia was prepared in 1774 by Joseph Priestly, and its composition
was determined in 1785 by Claude-Louis Berthollet. Ammonia has a chemical
formula of NH3, and is sp3 hybridised. Ammonia is highly
polarised, due to the electronegativity of nitrogen, and as a result, has a
large dipole moment. Ammonia
NH3 (aq)
+ H2O (l) Û NH4 (aq) + OH (aq)
Ammonia
solutions are basic, due to the hydroxide ions formed in solution.
Ammonia
is commercially produced by the Haber-Bosch process, which is also sometimes
referred to as the
Left: Fritz Haber, 12.9.1868 - 29.1.1934. Right: Carl Bosch, 27.8.1874 Both images taken without consent from


So how did the Haber-Bosch
process come about?
At
the end of the 19th century, Chilean nitrates, were the major source
of nitrates at the time. It was clear, that this source would not be able to
meet future demands. It was also realised that in the event of a war, any
nation cut off from the Chilean supply, would not be able to make adequate
amounts of munitions. Germany (Haber
So what is the Haber-Bosch process?
The
production of ammonia is achieved by the direct combination of hydrogen and
nitrogen, over an iron or aluminium catalyst. Hydrogen is obtained from the
decomposition of methane by heating. Nitrogen is obtained from the distillation
of liquefied air. It was the first chemical process to use high-pressure
conditions. The reaction is shown below:
N2(g)
+ 3H2(g) Û 2NH3(g)
The
process is highly exothermic with a ÙH value of
(LeChetelier
Catalyst:
The catalyst
provides an alternative pathway for the reaction to occur, which has lower
activation energy. This means that a lower temperature can be used without
compensating the rate too much. Carl Bosch found that a mixture of Fe2O3 and Fe3O4
catalyses the reaction best over the temperature range 650
Pressure:
Also due to
LeChetelier
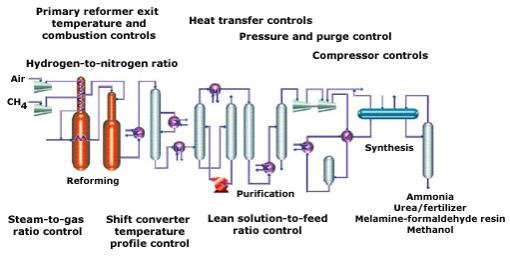
Below is a
diagram of a modern ammonia production site:
Taken from
What is ammonia used for?
Ammonia is a very useful product indeed. There are many, many uses for
ammonia and it
More information on
ammonia related subjects:
http://www.Foxboro.com/industries/ammonia
http://www.analyticpower.com/NH3CRACK.html
Some useful information:
Click here to link to my home
page
Click here if you wish to e-mail
me