Introduction
So who was he?
What are Van der
Waals forces?
What's his equation
all about?
Get to the lizards already
Other stuff
Links and references
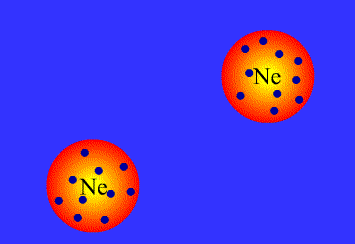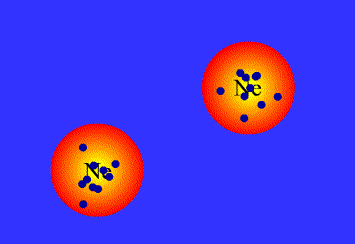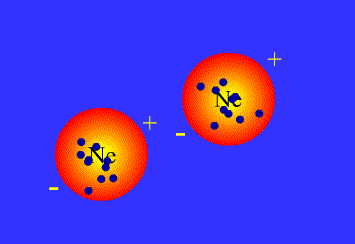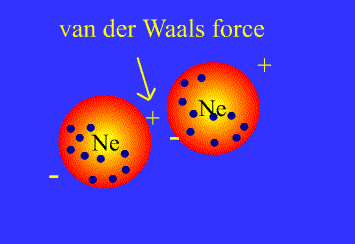
What are Van der Waals forces?
There is some confusion about this in A-Level syllabuses, as generally people are told that Van der Waals forces arise from the interactions between temporary dipoles in electron clouds, and have nothing to do with permanent dipoles or hydrogen bonds. In fact, all intermolecular attractions are known collectively as Van der Waals forces. Each force was discovered and explained at a different time, and so have separate names. This page covers:
-
London, or dispersion forces (formerly known as Van der Waals forces)
-
Keesom forces (formerly known as dipole-dipole interactions)
Both these forces are weak in themselves, but they can significantly alter properties such as melting and boiling points. The greater the interactions between atoms the higher the melting and boiling points, as more energy is required to break separate the atoms in the material in solid or liquid state.
Keesom forces
These are heavily related to the electronegativity of an atom. Electronegativity can be defined as the ability of an atom to draw electron density to itself within a molecule. Therefore more electronegative atoms are more electron rich and have a δ- charge. This type of dipole is permanent, which distinguishes this type of force from London force above. This time the strength of the force is related to the difference in electronegativity, or ΔEN between the two ends of the molecule, or between the two atoms in a simple diatomic molecule.