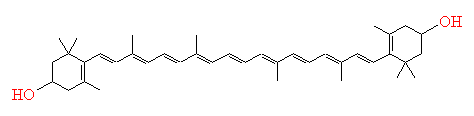
Carotene
 Carotene is a precursor to vitamin
A, and is the molecule that is found in carrots which helps to give them
their orange colour. The molecule is a highly conjugated system, with a series of
11 conjugated double bonds joined into a stiff straight backbone. This gives rise
to two intense absorptions in the blue-green region of the spectrum (lmax
= 451 nm). The longer wavelengths of red and yellow light are reflected, and so to
the human eye, carotene appears orange.
Carotene is a precursor to vitamin
A, and is the molecule that is found in carrots which helps to give them
their orange colour. The molecule is a highly conjugated system, with a series of
11 conjugated double bonds joined into a stiff straight backbone. This gives rise
to two intense absorptions in the blue-green region of the spectrum (lmax
= 451 nm). The longer wavelengths of red and yellow light are reflected, and so to
the human eye, carotene appears orange.
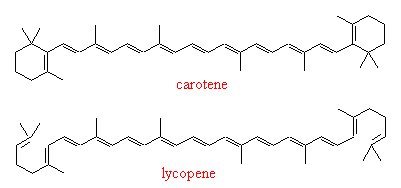
Carotene as Nature's pigment
 Carotene, and carotene-like compounds (carotenoids),
appear as pigments in many places in Nature. As well as carrots (whose yellow core
is actually due to oxidised versions of carotene called xanthophylls), it is partially responsible for the colour of mangos, the pale cream of milk and the yellow of butter. In fact, carotene anologues are added to margerine to make it look like butter. Tomatoes and other ripe red/yellow fruits owe their colour
to carotenoids, such as lycopene.
Carotene, and carotene-like compounds (carotenoids),
appear as pigments in many places in Nature. As well as carrots (whose yellow core
is actually due to oxidised versions of carotene called xanthophylls), it is partially responsible for the colour of mangos, the pale cream of milk and the yellow of butter. In fact, carotene anologues are added to margerine to make it look like butter. Tomatoes and other ripe red/yellow fruits owe their colour
to carotenoids, such as lycopene.
An Aid to Photosynthesis
Carotene is closely allied to chlorophyll
in plants and organisms that undergo photosynthesis. It serves two roles. First it
absorbs some of the sunlight that is missed by the chlorophyll molecules. Secondly,
because of its high number of reactive double bonds, it makes a good 'sponge' to
react with, and therefore 'mop up', energetic oxygen molecules that might otherwise
oxidise and degrade more important parts of the plant structure. In a typical leaf,
there is a ratio of chlorophyll to carotenoid of about 1:3, and the darker the leaf
the more carotenoids it contains. Normally, however, the green colour of chlorophyll
is so intense it masks the more subtle oranges and reds of the carotenoids. In Autumn
however, when the chlorophyll decays, the green fades and leaves turn red and yellow.
 | As the chlorophyll in leaves decays in the autumn, the green colour fades and is replaced by the oranges and reds of carotenoids.
|
Other Carotenoids
Some meat fats often have a slight yellow tint due to them having dissolved some of the carotene that the animal had extracted from the plants it had eaten. Carotenoids, especially those which contain oxygen groups (the xanthophylls) also occur in bacteria, fungi and marine biology, for example in the skins of fish, sea stars,
anemones, corals and crustaceans. The carotenoid is often bound to a protein which
modifies its colour slightly. This protein can be denatured by placing it boiling
water. When this happens, the carotenoid is freed and its colour re-emerges - for
example, in the red colour of boiled lobster. Examples of xanthophylls are zeaxanthin, which is responsible for the yellow of corn, mango, egg yolk and orange juice, and astaxanthin, which gives the pink colour to salmon, shellfish, shrip and lobster. Astaxanthin minus its two -OH groups is called canthaxanthin, which gives the flamingo its pink colour. Indeed, flamingos in captivity that are not given a regular diet of astaxanthin-containing shrimp soon lose all their colour and turn white!
References:
- Introduction to Organic Chemistry, Streitweiser and Heathcock (MacMillan,
New York, 1981).
- Molecules, P.W. Atkins (W.H. Freeman and Co, New York, 1987).
 Carotene is a precursor to vitamin
A, and is the molecule that is found in carrots which helps to give them
their orange colour. The molecule is a highly conjugated system, with a series of
11 conjugated double bonds joined into a stiff straight backbone. This gives rise
to two intense absorptions in the blue-green region of the spectrum (lmax
= 451 nm). The longer wavelengths of red and yellow light are reflected, and so to
the human eye, carotene appears orange.
Carotene is a precursor to vitamin
A, and is the molecule that is found in carrots which helps to give them
their orange colour. The molecule is a highly conjugated system, with a series of
11 conjugated double bonds joined into a stiff straight backbone. This gives rise
to two intense absorptions in the blue-green region of the spectrum (lmax
= 451 nm). The longer wavelengths of red and yellow light are reflected, and so to
the human eye, carotene appears orange.
 Carotene, and carotene-like compounds (carotenoids),
appear as pigments in many places in Nature. As well as carrots (whose yellow core
is actually due to oxidised versions of carotene called xanthophylls), it is partially responsible for the colour of mangos, the pale cream of milk and the yellow of butter. In fact, carotene anologues are added to margerine to make it look like butter. Tomatoes and other ripe red/yellow fruits owe their colour
to carotenoids, such as lycopene.
Carotene, and carotene-like compounds (carotenoids),
appear as pigments in many places in Nature. As well as carrots (whose yellow core
is actually due to oxidised versions of carotene called xanthophylls), it is partially responsible for the colour of mangos, the pale cream of milk and the yellow of butter. In fact, carotene anologues are added to margerine to make it look like butter. Tomatoes and other ripe red/yellow fruits owe their colour
to carotenoids, such as lycopene.