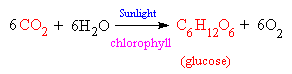
Provided sufficient oxygen is present, carbon dioxide, CO2, is the end product of the process of combustion of carbon based materials. If there is insufficient oxygen, carbon monoxide CO, is formed instead. Carbon dioxide is also the gas we exhale, since it is the product of respiration, whereby oxygen atoms are used to 'burn' (oxidise) the foodstuffs to release the energy necessary to sustain life. Carbon dioxide is a low energy compound, that is, its formation corresponds to the maximum release of energy. This means that CO2 is relatively inert, since a lot of energy needs to be used to persuade it to react to form a higher energy compound. However, Nature has found a way to get around this problem, and make use of CO2 by use of a catalyst (chlorophyll) and the energy from sunlight. This process, photosynthesis, which occurs in all green plants, converts CO2 and water to carbohydrates, releasing oxygen as a waste product.
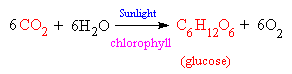
CO2 is a colourless, odourless gas under normal conditions, although if cooled to below -78.5°C it solidifies to form 'dry ice', which finds use as a refrigerant or for 'smoke' effects at rock concerts. It exists as a linear molecule of structure O=C=O. It can be made in the laboratory by the action of heat or acids on carbonates. Indeed, it is the action of acid rain on limestone (calcium carbonate) rocks, which releases CO2, and creates holes in the rocks which eventually form caves and caverns.
 | The White Cliffs of Dover, made of limestone. |
Fermentation of carbohydrates also produces CO2, and this incomplete form of respiration produces alcohol as a by-product. The bubbles in champagne, or in the head of beer are CO2, since this gas comes out of solution when the bottle is opened and the pressure released. Carbon dioxide is often deliberately added to various beverages to add the sparkle to fizzy drinks. In water, CO2 dissolves to form carbonic acid, which is a very weak acid with mild bactericide properties. Carbonic acid also has a number of other beneficial effects, such as enhancing taste, encouraging digestion, as well as giving a pleasant tingling sensation on the tongue.
Carbon dioxide is also used in baking. Typical baking powders consist of a number of components, including sodium bicarbonate (NaHCO3). They also contain a mild acid, such as tartaric acid, an acidic salt (sodium aluminium sulphate) and starch. The real trick to baking is to create the correct chemistry such that CO2 is released at two separate times throughout the bake cycle. The first occurs at room temperature, when the moist acid slightly reacts with the carbonate to release CO2 and so produce many tiny cavities in the batter. The second occurs in the oven, and is due to the action of the aluminium salt, which being less acidic only reacts at higher temperatures. The CO2 released in this second process swells the cavities to give the bread its light, fluffy texture. In a similar way, CO2 is also used to make bread, but in this instance the CO2 comes from yeast, a culture which feeds on sugar and other small carbohydrates.
 Carbon dioxide has the property that it is transparent to visible light, but blocks infra-red. This means that visible light from the sun penetrates through the atmosphere to reach the Earth's surface, which warms up. As it warms, it emits infra-red radiation (heat) which is emitted back into the atmosphere, but cannot escape back into space because the CO2 in the atmosphere blocks it. Thus CO2 has the effect of trapping heat, and thus keeping the Earth's surface warm. This is a similar process to that seen in greenhouses, so has been called the 'Greenhouse Effect'. So the concentration of CO2 in the atmosphere is very important for controlling how hot the Earth becomes. For the past several million years, this concentration has remained relatively constant (for the reasons mentioned earlier), but in the last 90 years or so, burning of fossil fuels (petrol, oil, natural gas) to heat our homes and power our cars has resulted in a large increase in the amount of CO2 being dumped into the atmosphere. If the Earth's natural processes (such as dissolving it into the oceans) cannot maintain the equilibrium, the level of CO2 may reach levels at which the Greenhouse Effect becomes a problem. In the worst scenario, this would lead to an increase in the average temperature at the Earth's surface of a few degrees - a process known as 'Global Warming'. This could cause widespread melting of the icecaps, with a corresponding increase in the sea-level around the world, leading to flooding and submergence of large areas of low-lying land and many coastal regions. It might also lead to millions of acres of fertile land turning into deserts, resulting in widespead famine and hunger. However, this is by no means definite - many alternative theories predict that the global warming is not as big problem as some pessimists make out. Nevertheless, many scientists are engaged in research around the world to try to determine how significant the Greenhouse Effect will be for people living in the next 50 years.
Carbon dioxide has the property that it is transparent to visible light, but blocks infra-red. This means that visible light from the sun penetrates through the atmosphere to reach the Earth's surface, which warms up. As it warms, it emits infra-red radiation (heat) which is emitted back into the atmosphere, but cannot escape back into space because the CO2 in the atmosphere blocks it. Thus CO2 has the effect of trapping heat, and thus keeping the Earth's surface warm. This is a similar process to that seen in greenhouses, so has been called the 'Greenhouse Effect'. So the concentration of CO2 in the atmosphere is very important for controlling how hot the Earth becomes. For the past several million years, this concentration has remained relatively constant (for the reasons mentioned earlier), but in the last 90 years or so, burning of fossil fuels (petrol, oil, natural gas) to heat our homes and power our cars has resulted in a large increase in the amount of CO2 being dumped into the atmosphere. If the Earth's natural processes (such as dissolving it into the oceans) cannot maintain the equilibrium, the level of CO2 may reach levels at which the Greenhouse Effect becomes a problem. In the worst scenario, this would lead to an increase in the average temperature at the Earth's surface of a few degrees - a process known as 'Global Warming'. This could cause widespread melting of the icecaps, with a corresponding increase in the sea-level around the world, leading to flooding and submergence of large areas of low-lying land and many coastal regions. It might also lead to millions of acres of fertile land turning into deserts, resulting in widespead famine and hunger. However, this is by no means definite - many alternative theories predict that the global warming is not as big problem as some pessimists make out. Nevertheless, many scientists are engaged in research around the world to try to determine how significant the Greenhouse Effect will be for people living in the next 50 years.