|
|
 |
 |
|
|
|
|
Molecules containing rings are extremely important in chemistry and biology. The simplest are just the hydrocarbon alkanes, which have been bent around so the ends meet. The first of this series is cyclopropane, with the C-C bond angles being only 60°. This causes a great deal of strain within the molecule, and it is no surprise that cyclopropane is very reactive. The next in the series is cyclobutane, which is also highly strained. Cyclopentane is slightly more stable because it can relieve some of the bond strain by buckling the ring and pushing one carbon out of the plane. Cyclohexane has effectively zero strain, since all the carbons now have exactly the correct bond angle for optimum overlap of their orbitals with their neighbours.
|
|
 |
 |
|
|
|
|
 |
 |
|
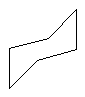
boat form
| chair form |
Cyclohexane is commonly found in two versions, one resembling a chair and one resembling a boat shape. The chair form has slightly less energy than the boat form, and so is the preferred conformer. With higher cycloalkanes, the strain increases again since there begins to be some eclipsing of adjacent pairs of C-H bonds, or bumping together of hydrogens across the ring. But at even larger ring structures (above about 16 carbons), even these effects become negligible and the rings are strain free.
 |
 |
|
|
|
 |
 |
|
|
|
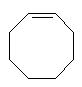
Ring structures also exist which include some of the C-C double bonds. The smaller cycloalkene rings are even more strained and reactive than the corresponding cycloalkanes.
 |
|
 |
|
|
|
|
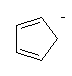
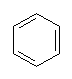
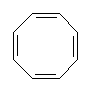
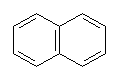
With more double bonds, the rings become more planar, but with increased chance of orbital overlap the structures can become more stable. Some structures, where the overlap is perfect are exceptionally stable, and these are called aromatic. Cyclobutadiene is the first member of the series to show enhanced stability due to this resonance effect. The next member, cyclopentadiene, does when it is a negative ion, and it is used as a ligand in many important organometallic chemicals, for example ferrocene. The most well-known aromatic compound is benzene, but higher mass aromatics also exist, for example, cyclooctatetrane.
 |
 |
 |
 |
|
|
|
|
|
 |
 |
|
|
|
 |
 |
|
|
|
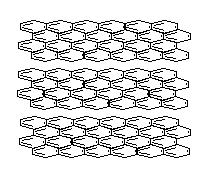
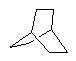
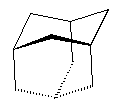
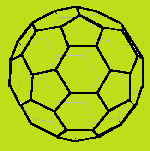
With enough carbon atoms, it becomes possible to make rings that are joined together in a non-planar fashion, such as bicyclo-3,3,1-octane. It is even possible to make interconnected rings, such as bicyclo-2,2,2-octane or adamantane (the building block of diamond). Perhaps the ultimate 3D ring structure is C60, the first of the fullerenes.
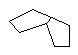 |
 |
|
|
|
 |
 |
|
|
|