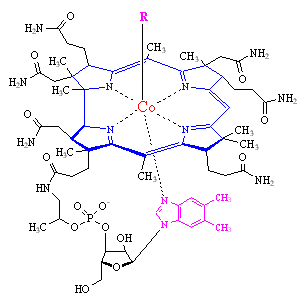
Vitamin B12 The corrin ring is shown in blue, the 5,6-dimethylbenzimidazole is in magenta, and the central cobalt atom is shown in red. In the commercial form, R = CN, and in the biologically active form, R = adenosine.
In 1926 it was discovered that pernicious anemia could be overcome if the patient ate large amounts of liver, led chemists to begin the search for the active agent. This curative factor was found and isolated in 1948 and given the name Vitamin B12. The complete three-dimensional structure of this complicated molecule wasn't obtained, however, until 1956, through the pioneering X-ray studies of Dorothy Hodgkin (Bristol University??) - who obtained the Nobel prize for this work in 1964. Vitamin B12 was synthesised 16 years later by R.B. Woodward (Harvard University) and A. Eschenmoser (Swiss Federal Institute of Technology). The synthesis took 11 years and involved more than 90 separate reactions performed by over 100 co-workers.

Vitamin B12 The corrin ring is shown in blue, the 5,6-dimethylbenzimidazole is in magenta, and the central cobalt atom is shown in red. In the commercial form, R = CN, and in the biologically active form, R = adenosine.
Vitamin B12 is the only known biomolecule with a stable carbon-metal bond. The core of the molecule is a corrin ring with various attached sidegroups. This ring consists of 4 pyrrole subunits, the nitrogen of each of which is coordinated to a central Co atom. In the stable commercial form of the molecule, the cobalt (in its +3 oxidation state) is bonded to a CN group. The sixth ligand below the ring is a nitrogen of 5,6-dimethylbenzimidazole.
The cobalt of vitamin B12 can be reduced by biochemical reactions with the body to a +2 or +1 oxidation state. In the +1 state, the molecule is called vitamin B12s and becomes one of the most powerful nucleophiles known. It is more nucleophilic than methanol by a factor of 1014. Vitamin B12s can react with adenosine triphosphate (ATP) to yield the biologically active form of the vitamin, where the cyano group is replaced by and adenosine group.
More information of the structure and component parts of vitamin B12 can be found as a Chime-enhanced page on the Oxford MOTM site.
John Maher, Paul May, Bristol University 1997.