 | A tropical island in the Pacific Ocean, surrounded by deep blue water. |
Water is the most common substance on Earth, and it undoubtedly has the most well-known of all chemical formulas: H2O. In fact it is only one of 2 liquids which occur naturally on the planet in appreciable quantities (the other is petroleum). More than three-quarters of the Earth's surface is covered in water, and 60% of the human body weight is water. The central role played by water in all life processes was obvious from the earliest times, and Aristotle regarded it as one of the four 'elements' (along with fire, earth and air).
The water molecule is composed of just 2 hydrogens and one oxygen and this small molecule is bent into a V-shape. In spite of this apparent simplicity, water is a highly unusual compound. It has many special qualities which enable it to play its vital role in inorganic and biological systems. It is an almost universal solvent, and mixes well with many organic substances that can form hydrogen bonds, such as alcohol (in beer, wine and spirits), and sugar (as in most sweet drinks). It also dissolves many ionic solids, such as salts and other minerals to produce the salty chemical soup of the sea.
 | A tropical island in the Pacific Ocean, surrounded by deep blue water. |
Water is also essential for life, since it provides the correct medium for the transport of nutrients into, and waste products out of, cells. Water acts as the environment between and around cells through which other molecules can move. One obvious example is blood, which is largely water, and which acts as the transportation system for almost all of the body's essential chemicals.
Despite water's ubiquity, its chemical structure did not become evident until the late 18th century, since it had to first wait for the discovery of its component elements, hydrogen (discovered by Henry Cavendish in 1766) and oxygen (discovered by Joseph Priestley in 1774). Armed with this new information, Cavendish burnt measured quantities of these 2 gases and found that the only reaction product was water. Antoine Lavoisier then confirmed this by decomposing water back into H2 and O2, and finally in 1871 Cannizzaro established the formula to be H2O.
Pure water is not colourless. It is actually pale blue, although this colour is not apparent unless more than 2 metres of it is viewed against a white background, in for example, a swimming pool. The same pale blue can often be seen in some ice formations. The reason for this colour is due to a slight absorption of red light, caused by the hydrogen bonds modifying the normal vibrational frequency of the water molecule.
The structure of liquid water is much more complex than it might appear. This is due to the tendency for the bent H2O molecules to form strong hydrogen bonds with their neighbours. Experiments at room temperature have indicated that a water molecule oscillates within a surrounding cage of other molecules for about 4 ps, before jumping out of this position and into an adjacent cage. The complexity arises from the fact that the water molecules can also spontaneously break apart to form H+ and OH- ions. The average spacing between hydrogen and hydroxyl ions is about 400 water molecules, although they are both continually moving around within the whole ensemble of molecules.
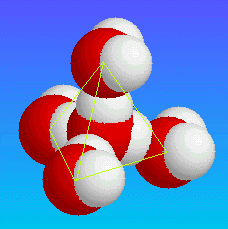 | The hydrogens on each water molecule point toward the oxygens on neighbouring water molecules, such that the whole structure is based around a tetrahedral shape. Although since this is a liquid, the molecules are continually moving and rotating, breaking and reforming hydrogen bonds, so the tetrahedral structure can be thought of as only a time averaged approximation. |
Under normal conditions on Earth, water is a liquid, and it freezes at an unexpectedly high temperature due to the strong hydrogen bonds. Water is also very odd in that it is one of the only substances known where the liquid form is denser than the solid form. In fact, water is at its most dense at 4°C, and the result of this is that ice floats on water rather than sinking to the bottom. This allows icebergs to form, and also creates the solid ice-sheets on top of frozen ponds and lakes. This layer of insulation can prevent the entire mass of liquid below from freezing solid, so allowing aquatic life to survive the winter.
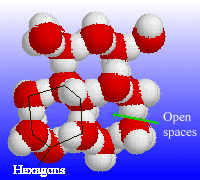 | The structure of a small portion of ice, showing the open spaces between rows of hexagons. |
The reason for the anomalously low density of ice results from its structure. At low temperatures the water molecules align themselves using intermolecular hydrogen bonds to form an open, hexagonal structure. This structure has many gaps and spaces, and is therefore less dense than the corresponding liquid structure. The hexagonal structure is also responsible for the 6-fold symmetry of snowflakes.
Under normal conditions, water boils at 100°C. Indeed, the Centigrade scale by which we measure temperature is based upon dividing the temperature difference between the boiling point and the freezing point of water into 100 equal units. When water boils and turns into steam, the hydrogen bonding is broken and the individual molecules vaporise. This causes a large change in volume, which can be used to perform mechanical work, such as moving a piston. This simple process became the driving force for the Industrial Revolution, which issued in the era of the steam engine, the factory, and all the benefits (and problems) of the industrialised world we now live in.