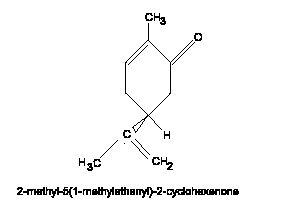
The above diagram is that of Carvone. "So what!" you might think. As it happens Carvone is a particularly good demonstration model for Chiral compounds. In the form shown it would smell like caraway seeds. If, however, you had the other enantiomer, with the doubly bonded oxygen and double bond swapping sides, then its smell would be completely different, in fact you would think that it was spearmint!!!
Our noses are very clever. The receptors on the surface are in fact chiral, and so make it easy to distinguish between different smells by their orientation.
You have to be very careful with which enantiomer you use. The popular drug from the 1960's, Thalidomide, was a chiral compound. It was used as a sedative, but was found to cause deformities in children. Sadly it was given as a racemic mixture, as one of the enantiomers caused the deformity.
This is the case in a number of medicines; only one enantiomer is biologically active, the other does nothing.
Unfortunately you will need the chemical/x-pdb plug in for netscape, see their homepage.