Diamond.

Diamond is a rarer allotrope of carbon, it is very expensive and very sought after. For years people have done many things
to get diamond, they have stolen, the have killed to get their hands on it. Click HERE for a
quick history of diamond. (Credit to Dr. May's Molecule of the month page) Mythology has always surrounded diamond, it plays a large part
in religions and cultures around the world.
Extraction of diamond
Diamonds although rare occur naturally. They are mined in South Africa and Brazil. They have also been found in
metorites hitting the Earth. It is also possible to make Diamonds synthetically, in fact
in Bristol University's Chemistry department there is a research group that do just that

Click on the above picture to see the results of the latest research.
The group are engaged in creating synthetic diamond coatings on common materials such as wires, this increases their strength.
To see an electron micrograph photo of diamond film deposition, click HERE
Synthetic diamonds have been made since the early 1950's, by a process called High Pressure High Temperature synthesis (HPHT).
This is an attempt to mimic the conditions under which natural diamond forms deep in the earth.
Graphite is put into a huge hydraulic press at high temperatures and pressures, and with the addition of a metallic catalyst,
converts to diamond over a period of a few hours. The diamond crystals that are produced by this method are typically a few mm in size,
which are too flawed for use as gemstones, but are extremely useful as hard-wearing edges on cutting tools and drill-bits.
The structure of Diamond.
The structure of diamond is very strong, in fact diamond is the hardest material known to man. This is because of its structure.
The structure is rigid, every carbon atom is joined to 4 neighbouring carbon atoms by single sigma bonds.
Because sigma bonds are so strong it is not possible to move the structure very easily, and therefore it is very strong.
See the diagram below:
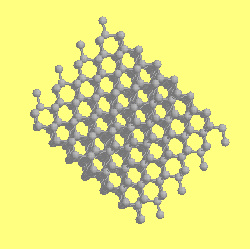
The structure of diamond
What are diamond's uses?
When we talk of diamonds we always think of thos pretty little (or large) gemstones which are put in to rings.
Diamond due to its high reflectivity and high refractive index sparkles and is very beautiful. This high refractive index
has other potential applications, for example the higher index a lens is the thinner it could potentially be
, if synthetic production of optical quality diamonds becomes economically viable there could be a very useful
cosmetic alternative to thick lenses in glasses? Also materials with high refractive indicies could be useful
for other optical equipment.
Diamond's hardness is also an important property, as it is used in tools which have to cut through materials.
Diamond is very hard wearing, and lasts for a long time on for example a drill bit compared to other materials.
In fact I myself have used a diamond tool when I worked at a well known optical company, I used it for surfacing glass lenses.
Click here to go back to the carbon home page