
In general PVC is formed when individual vinyl chloride (chloroethene) monomers polymerise to form polyvinyl chloride, as in the following reaction scheme:
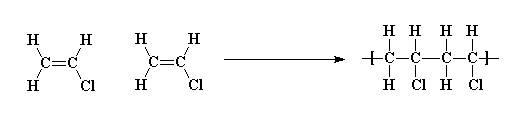
| Record chemistry - PVC |
 |
||||||||||
|
Polyvinyl chloride is, as the name suggests, a vinyl polymer. It is also one of the most common man made polymers in existence. PVC can be found in just about every house in the western world, every person reading this will come in contact with it today, if they haven't already. In general PVC is formed when individual vinyl chloride (chloroethene) monomers polymerise to form polyvinyl chloride, as in the following reaction scheme:
| |||||||||||
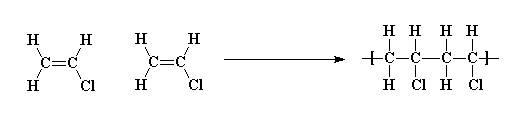
|
|||||||||||
|
The polar Cl-C bond results in a strong dipole that produces considerable intermolecular forces between chains. This results in pure PVC having a very rigid structure and it is this form (slightly modified) that is used in pipes, 'gramophone' records and consumer goods. Plasticiers can be added to PVC to make it into highly flexible forms used in Wellington boots etc.
|
|||||||||||
|
back to main chemistry page |
|||||||||||
| Front | Chemistry | History | How it works | Make your own | Links |
| dcressey@another.co.uk | copyright Daniel Cressey 2001 |