

Mendeleyev
For years there has been some disagreement between scientists about who is the real founding father of the Periodic table, the German Lothar Meyer or the Russian Dmitri Mendeleyev. Both produced very similar results at the same time even though the two scientisits worked on thier own. The deciding factor was that Mendeleyev's periodic table became avaliable before Meyer's!
Mendeleyev was the youngest of 17 children and came from the Siberian town of Tobolsk. In his early days he wasn't considered to be an outstanding student and this factor along with his Siberian background he was denied admission to the top universities in Russia. After impressing with his science teaching skills (in a gymnasium!) he was finally admitted to St. Petersburg University where he later became a professor, after furthering his studies in Germany.
While writing a textbook on systematic inorganic chemistry, Principles of Chemistry, he organised his material into "combining power" or valency along with the "family" method which was based on the same principles explained through the laws of triads and octaves. However he found problems with elements such as copper and mercury which changed their combining power depending on what it was bonded to. While trying to solve this problem he noticed property patterns between the alkali metals, the alkali earth metals and the Halogens. To extend these patterns he created a series of cards, one for each element, and arranged them in order of ascending atomic weight, then grouping elements according to their similar chemical properties. This led to an "Element Solataire" as the arrangement of cards resembled his favourite card game. The Periodic Table was born and led him to create the periodic law.
Mendeleyev's Periodic Table of 1869
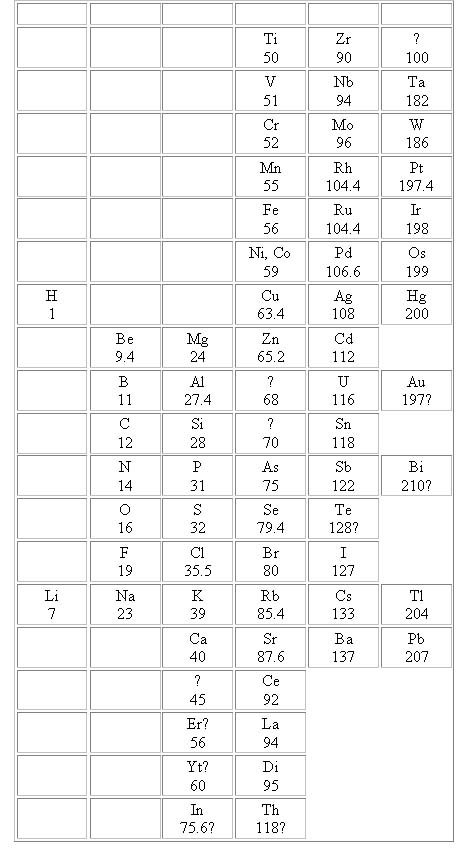
When this table was published in 1869 it came with a statement for Periodic
Law, this contained 8 statements:
1. The elements, if arranged according to their atomic weights
show an evident periodicity of properties.
2. Elements which are similar as regards their chemical properties have atomic
weights which are either nearly the same value or which increase regularly.
3. The arrangement of the elements or of groups of elements in the order of
their atomic weights, corresponds with their so called valencies.
4. The elements which are most widely distributed in nature have small atomic
weights, and sharply defined properties. They are therefore typical elements.
5. The magnitude of the atomic weight determines the character of an element.
6. The discovery of many as yet unknown elements may be expected.
7. The atomic weight of an element may sometimes be corrected by the aid of
a knowledge of those of adjacent elements.
8. Certain characteristic properties of the elements can be foretold from
their atomic weights.
From this statement he predicted the existence of yet unknown
elements, many of which were confirmed before his death.