Introduction
 Haemoglobin
is one of the two oxygen binding proteins found in vertebrates. It's function is
to carry oxygen in the blood from the lungs to other tissues in the body, in
order to supply the cells with the oxygen required by them for oxidative
phosphorylation of foodstuffs. Haemoglobin is found in the blood within
the erythrocytes (red blood cells). These cells essentially act as a sack
for carrying haemoglobin, since mature erythrocytes lack any internal organelles
(nucleus, mitochondria, etc). The other oxygen binding protein found in vertebrates
is myoglobin, which stores the oxygen in the tissues of the body
ready for when the tissues require it. The highest concentration of myoglobin
are found in skeletal and cardiac muscle which requires large amounts of oxygen
because of there need for large amounts of energy during contraction.
Haemoglobin
is one of the two oxygen binding proteins found in vertebrates. It's function is
to carry oxygen in the blood from the lungs to other tissues in the body, in
order to supply the cells with the oxygen required by them for oxidative
phosphorylation of foodstuffs. Haemoglobin is found in the blood within
the erythrocytes (red blood cells). These cells essentially act as a sack
for carrying haemoglobin, since mature erythrocytes lack any internal organelles
(nucleus, mitochondria, etc). The other oxygen binding protein found in vertebrates
is myoglobin, which stores the oxygen in the tissues of the body
ready for when the tissues require it. The highest concentration of myoglobin
are found in skeletal and cardiac muscle which requires large amounts of oxygen
because of there need for large amounts of energy during contraction.
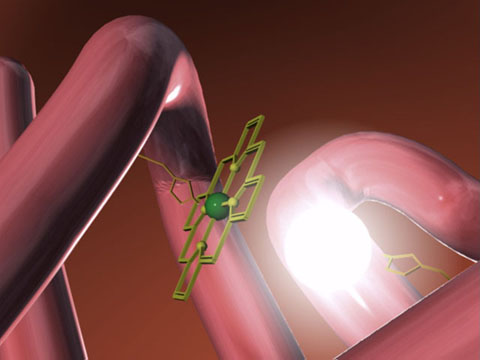
Mygolobin is the simpler of the two oxygen
binding proteins. It is made up of 153 amino acids in a single polypeptide
chain, and it was the first protein to have its three dimensional structure
determined by x-ray crystallography. Within a hydrophobic crevice of the
globular protein, formed by the folding of the poly peptide chain is the heme
prosthetic group (shown in the picture above, the polypeptide part shown in
pink, and the heme group shown in green). The heme group has a central iron atom
which is essential in the binding of oxygen.
Haemoglobin is essentially composed of four
polypeptide peptide units that are very similar to myoglobin.
On this web site the structure, and chemistry
of the heme group (responsible for the binding of oxygen) and of the actual
polypeptide will be discussed, along with a brief look at other oxygen binding
proteins found in nature.
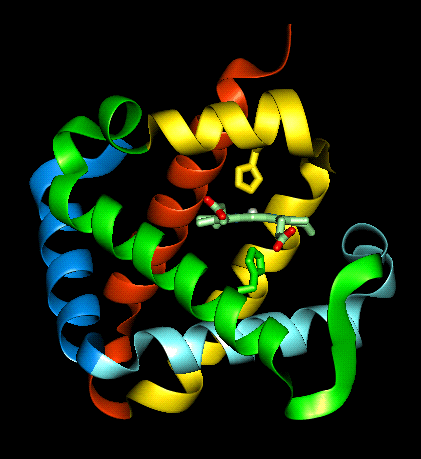 This
diagram shows one molecule of myoglobin, haemoglobin is made up of four of these
units bound together in a tetrahedron, via intermolecular forces to give the
quaternary structure (which will be discussed later).
This
diagram shows one molecule of myoglobin, haemoglobin is made up of four of these
units bound together in a tetrahedron, via intermolecular forces to give the
quaternary structure (which will be discussed later).
 Haemoglobin
is one of the two oxygen binding proteins found in vertebrates. It's function is
to carry oxygen in the blood from the lungs to other tissues in the body, in
order to supply the cells with the oxygen required by them for oxidative
phosphorylation of foodstuffs. Haemoglobin is found in the blood within
the erythrocytes (red blood cells). These cells essentially act as a sack
for carrying haemoglobin, since mature erythrocytes lack any internal organelles
(nucleus, mitochondria, etc). The other oxygen binding protein found in vertebrates
is myoglobin, which stores the oxygen in the tissues of the body
ready for when the tissues require it. The highest concentration of myoglobin
are found in skeletal and cardiac muscle which requires large amounts of oxygen
because of there need for large amounts of energy during contraction.
Haemoglobin
is one of the two oxygen binding proteins found in vertebrates. It's function is
to carry oxygen in the blood from the lungs to other tissues in the body, in
order to supply the cells with the oxygen required by them for oxidative
phosphorylation of foodstuffs. Haemoglobin is found in the blood within
the erythrocytes (red blood cells). These cells essentially act as a sack
for carrying haemoglobin, since mature erythrocytes lack any internal organelles
(nucleus, mitochondria, etc). The other oxygen binding protein found in vertebrates
is myoglobin, which stores the oxygen in the tissues of the body
ready for when the tissues require it. The highest concentration of myoglobin
are found in skeletal and cardiac muscle which requires large amounts of oxygen
because of there need for large amounts of energy during contraction. This
diagram shows one molecule of myoglobin, haemoglobin is made up of four of these
units bound together in a tetrahedron, via intermolecular forces to give the
quaternary structure (which will be discussed later).
This
diagram shows one molecule of myoglobin, haemoglobin is made up of four of these
units bound together in a tetrahedron, via intermolecular forces to give the
quaternary structure (which will be discussed later).