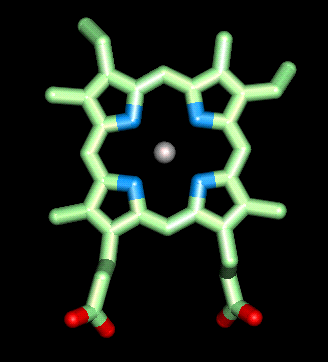
Structure and function of the heme group

The
heme prosthetic group in myoglobin and haemoglobin is made up of a
protoporporphoryn IX ring (shown in green) and an iron ion in the ferrous (Fe
II) oxidation state (shown in silver). This ferrous ion binds four nitrogen
atoms within the ring (shown in blue) and will form two additional bonds on
either side of the plane of the porphyrin ring. One of these bonds is to another
nitrogen atom on a histidine residue 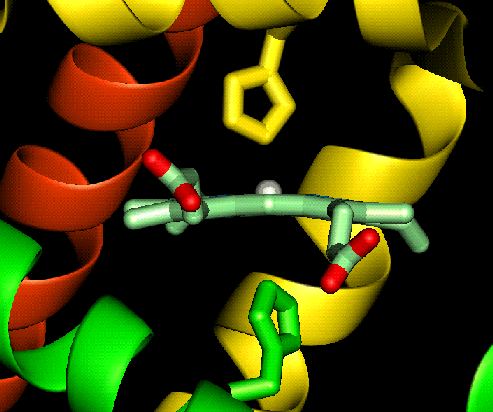 from
the actual polypeptide, and is called the proximal histidine (shown in yellow).
The sixth binding site is protected by another histidine residue called the
distal histidine (green). This serves two very important properties. First it
prevents the heme groups on neighboring haemoglobin molecules coming in to
contact with one another and oxidizing the ferrous ion to the ferric (III)
oxidation state in which it can no longer bind to oxygen. Secondly it
prevents carbon monoxide binding irreversibly to the ferrous ion in the heme
group, thereby lowering the affinity of the heme group for carbon monoxide. This
is important as once CO has bound irreversibly to the heme group it can no
longer bind oxygen.
from
the actual polypeptide, and is called the proximal histidine (shown in yellow).
The sixth binding site is protected by another histidine residue called the
distal histidine (green). This serves two very important properties. First it
prevents the heme groups on neighboring haemoglobin molecules coming in to
contact with one another and oxidizing the ferrous ion to the ferric (III)
oxidation state in which it can no longer bind to oxygen. Secondly it
prevents carbon monoxide binding irreversibly to the ferrous ion in the heme
group, thereby lowering the affinity of the heme group for carbon monoxide. This
is important as once CO has bound irreversibly to the heme group it can no
longer bind oxygen.
For example deoxy myoglobin (where we are
just concentrating on one heme group) is a five coordinate high spin ferrous
complex which also means that the complex is labile, and is capable of accepting
other ligands (oxygen) in the place of others (the distal histidine). Notice
that the ferrous ion is above the plane of the ring, this is due to the size of
the high spin ferrous ion. When oxygen completes the sixth coordination site the
ferrous ion changes to low spin and shrinks slightly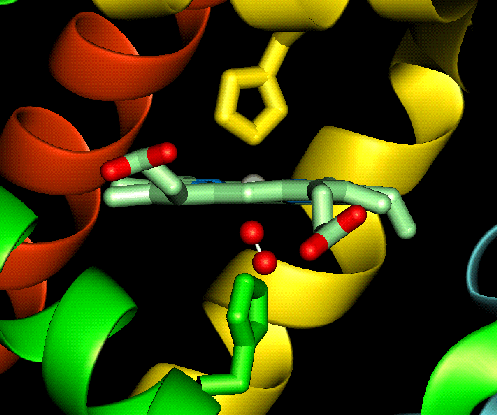 allowing the ion to move in to the plane of the ring (shown
below).
allowing the ion to move in to the plane of the ring (shown
below).
The effect of moving the ferrous ion in to the plane of the ring induces allosteric effects in haemoglobin (which will be discussed later). This is due to the fact that when the ferrous ion moves in to the plane of the ring it also pulls the proximal histidine along with it, therefore altering the shape of the polypeptide unit.
Thus, although the oxygen binding site in Haemoglobin and myoglobin is only a small part of the whole protein, the poly peptide chain modulates the function of the heme prosthetic group.