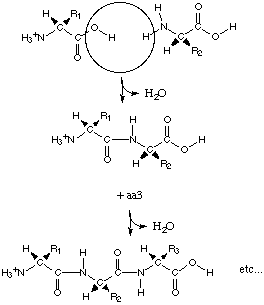
The Structure of the polypeptide
Both myoglobin and haemoglobin are typical
globular proteins and have intricate three dimensional structures. To rationalize
these intricate three dimensional structures the levels of organization in protein molecules have to be investigated. These levels are
primary, secondary, tertiary, and in the case of haemoglobin, quaternary levels
of organization.
The primary level of structure in a protein
is the linier sequence of amino acids, formed by a condensation reaction (and
therefore the abstraction of water) in protein synthesis. Each amino acid
residue is then bonded via peptide bonds. Other covalent bonds are also included
in the primary structure such as disulphide bonds.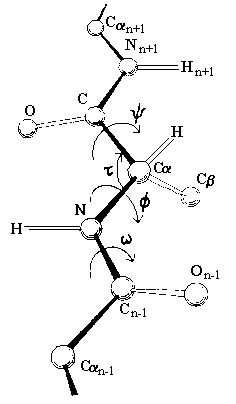
The peptide bond has partial double bond
character, and therefore the atoms in the peptide bond all lie in the same rigid
plane, called the amide plane. This restriction is important in defining the
folding of a protein in three dimensions. The only possible rotation of the main
chain is about the alpha carbon-carbon bond (designated the Greek letter psi),
and the alpha carbon-nitrogen bond (designated the Greek letter phi). Therefore
the amide planes define the polypeptide backbone.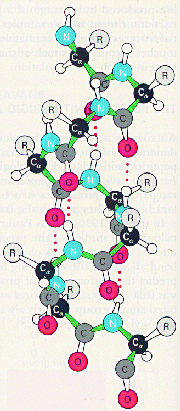
The secondary level of structure in a protein is the regular folding of the regions of the polypeptide chain. The two most common types are the alpha-helix and the beta-pleated sheet. Both myoglobin and haemoglobin are mostly made up from alpha-helices. In a rod like alpha-helix, the amino acids arrange themselves in a regular helical conformation. The carbonyl oxygen of each peptide bond is hydrogen bonded to the hydrogen on the amino group of the fourth amino acid away with the hydrogen bonds running nearly parallel to the axis of the helix. In an alpha-helix there are 3.6 amino acids per turn of the helix covering a distance of 0.54 nm, and each amino acid residue represents an advance of 0.15 nm along the axis of the helix. The side chains of the amino acids are all positioned along the side of the cylindrical helix.
 The
tertiary structure refers to the spatial arrangement of amino acids that are far
apart in the linear sequence as well as those residues that are adjacent. Again,
it is the sequence of amino acids that determine the three dimensional structure. in
water-soluble globular proteins such as myoglobin the main driving
force behind the folding of he polypeptide chain is the energetic requirement
to bury the non polar amino acids in the hydrophobic interior away from the
surrounding aqueous medium. Once folded the three dimensional, biologically
active (native) molecule is maintained not only by hydrophobic interactions, but
also by electrostatic forces, hydrogen bonding, and if present disulphide bonds.
The
tertiary structure refers to the spatial arrangement of amino acids that are far
apart in the linear sequence as well as those residues that are adjacent. Again,
it is the sequence of amino acids that determine the three dimensional structure. in
water-soluble globular proteins such as myoglobin the main driving
force behind the folding of he polypeptide chain is the energetic requirement
to bury the non polar amino acids in the hydrophobic interior away from the
surrounding aqueous medium. Once folded the three dimensional, biologically
active (native) molecule is maintained not only by hydrophobic interactions, but
also by electrostatic forces, hydrogen bonding, and if present disulphide bonds.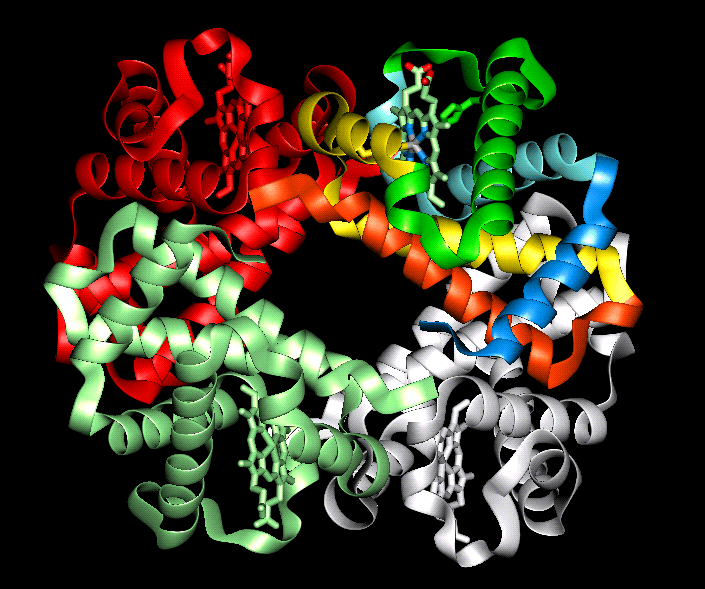
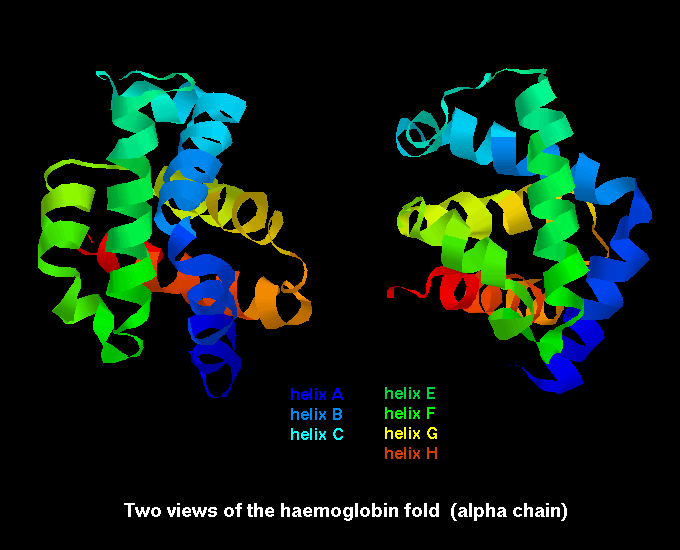
Proteins containing more than one polypeptide chain, such as haemoglobin exhibit a fourth level of protein structure called quaternary structure. This level of structure refers to the spatial arrangement of the polypeptide subunits and the nature of the interactions between them. In the case of haemoglobin the four subunits are held together by weak van der waals forces.