What is alcohol?
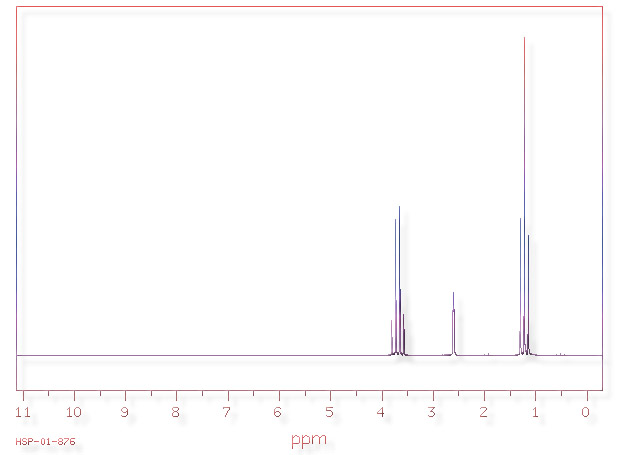
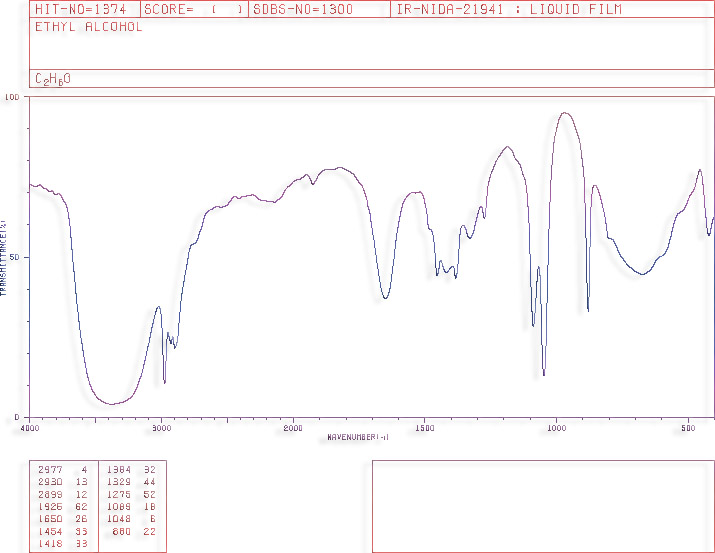
In a nutshell, alcohols are organic molecules containing -OH groups. The alcohol that is usually reffered to as 'alcohol' is ethanol, with formula CH3CH2OH. A model of ethanol is shown above.
The vital statistics:
-Clear, colorless
liquid
-Agreeable odor
-Dilute sweet flavor, concentrated burning taste
-Melting point: -114.11oC
-Boiling point: 78.51oC
-Density: .789 g/mL at 20oC
What it does to
you:
Ethanol acts as a drug affecting the central nervous system. It's behavioral
effects stem from its effects on the brain and not on the muscles or senses
themselves. Very low doses can appear to be a stimulant by suppressing certain
inhibitory brain functions. At higher doses further suppression of brain functions
produce the classic symptoms of intoxication: slurred speech, unsteady walk,
disturbed sensory perceptions, and inability to react quickly. Alcohol levels
in the brain are difficult to measure, and so blood alcohol levels are used
to assess degree of intoxication. Most people begin to show measurable mental
impairment at around 0.05 percent blood alcohol and above 0.5 percent blood
alcohol death occurs.
Humans have been making ethanol for thousands of years by the fermentation of sugars. All drinking ethanol, and half of all industrial ethanol are still made this way. The sugars from fruits and the starches from rice, wheat, potatoes and others can be used.
Ethanol's
IR spectrum (liquid film)

Ethanol's
1H NMR Spectrum