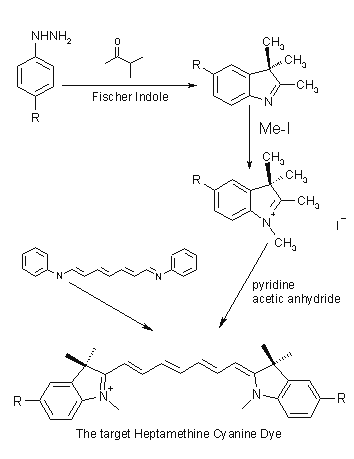
Dye Synthesis:
The synthesis of the exact dyes used in CD-R manufacture is unknown as the details are held under patent, but there are some general syntheses of cyanines and phthalocyanines available:
Cyanine
(polymethines):
A general synthesis of these materials is best outlined as a scheme for a heptamethine
dye.

A substituted hydrazine is condensed with methyl isopropyl ketone according
to the Fischer Indole Synthesis. N-methylation is achieved with methyl iodide
leading to an indolinium salt. The salts further react with N-[5-(phenylamino)-2,4-phenadienylidine]
aniline monohydrochloride, giving the cyanine dye with no substitution on the
conjugated chain.
Industrially, coal tar is used as a source for the heterocyclic precursors.
Phthalocyanine:
Pc
is incredibly simple to generate using a method of templated ring closure common
to many macrocyclic systems.
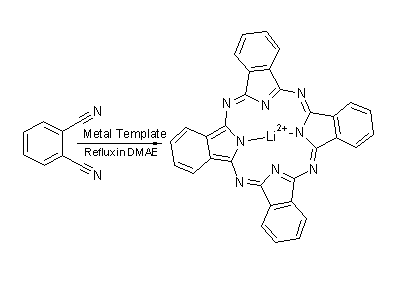
The starting material is 1,2-dicyanobenzene which is heated to reflux as a solution in N,N-dimethylaminoethanol (DMAE). In the presence of a metal to act as a template, yeilds are high and the reaction fast. The template is key and without it this reaction will not proceed at all. The nature of the metal is also important. If the metal ion template fits too well, it may prove impossible to remove from the Pc and the complex M-Pc may be the final product. Lithium is a good metal to use if the free phthalocyanine is required. The lithium ions in the centre of the Pc molecule can be removed simply by reaction with water. Of course, often a metal coordination is desired in the synthesis of a dye based on Pc, as we have already seen. If this is the case then the relevant metal can often be used as the template, saving a synthesis step and improving yields.