U

Uranium
hard, lustrous, silver-white, malleable and ductile, radioactive, metallic element of the actinide series, symbol U, atomic number 92, atomic weight 238.029. It is the most abundant radioactive element in the Earth's crust, its decay giving rise to essentially all radioactive elements in nature; its final decay product is the stable element lead. The chief ore is pitchblende(UO2), in which the element was discovered by German chemist Martin Klaproth 1789; he named it after the planet Uranus, which had been discovered 1781.
Uranium is an Actinide, situated in the bottom row of the periodic table. Uranium is readily available and relatively low in radioactivity. It can have oxidation state +3 through to +6 with +4 and +6 the most common. Uranium metal does not form a passive oxide of coatings hence it is corroded on prolonged exposure to air giving a complex mixture of oxides. Uranium Halides exist in the full range of oxidation states +3 to +6.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | |||||||||||||||||||
| 1 | 1 H |
2 He |
|||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
|||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
|||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
|
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
|
| 6 | 55 Cs |
56 Ba |
* | 71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
** | 103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Uun |
111 Uuu |
112 Uub |
113 Uut |
114 Uuq |
115 Uup |
116 Uuh |
117 Uus |
118 Uuo |
| *Lanthanoids | * | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
||||
| **Actinoids | ** | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
||||
The Uranium nucleus can be split apart. When this is done, a tremendous amount of energy is released. The energy is both heat and light energy. This energy, when let out slowly, can be harnessed to generate electricity. The word fission means to split apart.
![[ Photo of
Diablo Canyon Nuclear Plant ]](diablo.gif)
A nuclear power plant (like Diablo Canyon Nuclear Plant shown on the right) uses
uranium as a "fuel." Uranium is processed into tiny pellets that are
loaded into rods that are put into the power plant's reactor.
Inside the reactor of an atomic power plant, uranium atoms are split apart in a controlled chain reaction.
In a chain reaction, particles released by the splitting of the atom go off and strike other uranium atoms splitting those. Those particles given off split still other atoms in a chain reaction. In nuclear power plants, control rods are used to keep the splitting regulated so it doesn't go too fast.
Picture source: http://www.energy.ca.gov/education/story/story-images/diablo.gif
This chain reaction gives off heat energy. This heat energy is used to boil water in the core of the reactor. This water heats another set of pipes filled with water to make steam. The steam in this second set of pipes powers a turbine to generate electricity.

Uranium can also be used to make nuclear weapons. In August 1945 the first
Atomic Bomb was dropped on the Japanese city of Hiroshima killing 70,000 people.

The principle of nuclear weapons is the same as Nuclear fission with the exception that The Uranium must be compressed massively to produce the explosion, which results from a massive change in entropy.
Picture source: www.sunderlandfc.freeserve.co.uk
Uranium Extraction: Here is a few Photos of the the Elliot Lake Mine in Canada:

Inside the Mine:

Uranium Mining Techniques:
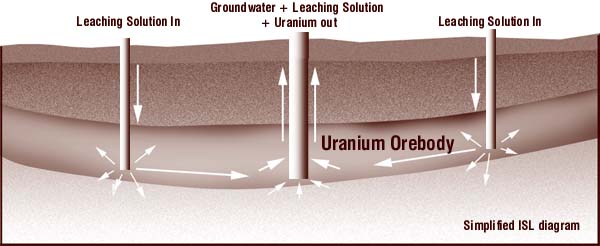
Traditionally, uranium has been extracted from open-pits and underground mines. In the past decade, alternative techniques such in-situ leach mining, in which solutions are injected into underground deposits to dissolve uranium, have become more widely used.
The milling (refining) process extracts uranium oxide (U3O8) from ore to form yellowcake, a yellow or brown powder that contains about 90 percent uranium oxide.
The most serious health hazard associated with uranium mining is lung cancer due to inhaling uranium decay products. Uranium mill tailings contain radioactive materials, notably radium-226, and heavy metals (e.g., manganese and molybdenum) which can leach into groundwater. Near tailings piles, water samples have shown levels of some contaminants at hundreds of times the government's acceptable level for drinking water.
At an In situ Leach site, a series of wells are drilled into the orebody. Millions of litres of strong acid or alkaline solution are then injected directly into the groundwater, stripping the uranium from the host rock and mixing it into the water. In the center of a circle of injection wells, a production well sucks most of the uranium bearing water up to the surface and pipes it into a processing plant where the uranium is recovered and the wastes pumped away.
Conversion and Enrichment
Uranium is generally used in reactors in the form of uranium dioxide (UO2) or uranium metal; nuclear weapons use the metallic form. Production of uranium dioxide or metal requires chemical processing of yellowcake. Further, most civilian and many military reactors require uranium that has a higher proportion of uranium-235 than present in natural uranium. The process used to increase the amount of uranium-235 relative to uranium-238 is known as uranium enrichment.
U.S. civilian power plants typically use 3 to 5 percent uranium-235. Weapons use "highly enriched uranium" (HEU) with over 90 percent uranium-235. Some research reactors and all U.S. naval reactors also use HEU.
To enrich uranium, it must first be put in the chemical form uranium hexafluoride (UF6). After enrichment, UF6 is chemically converted to uranium dioxide or metal. A major hazard in both the uranium conversion and uranium enrichment processes comes from the handling of uranium hexafluoride, which is chemically toxic as well as radioactive. Moreover, it reacts readily with moisture, releasing highly toxic hydrofluoric acid. Conversion and enrichment facilities have had a number of accidents involving uranium hexafluoride.
The bulk of waste from the enrichment process is depleted uranium--so-called because most of the uranium-235 has been extracted from it. Depleted uranium has been used by the U.S. military to fabricate armour-piercing conventional weapons and tank armour plating. It was incorporated into these conventional weapons without informing armed forces personnel that depleted uranium is a radioactive material and without procedures for measuring doses to operating personnel.
Go to: www.ieer.org
Other Uses of Uranium:
Go to www.webelements.com for more information.
All commercial reprocessing plants use the PUREX process, which has been proved over thirty years.
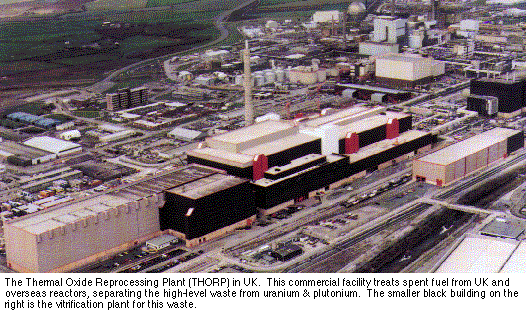
The spent fuel (which is highly radioactive) is chopped up and dissolved in hot concentrated nitric acid. The first stage separates the uranium and plutonium in the aqueous nitric acid stream from the fission products by a countercurrent solvent extraction process, using tributyl phosphate dissolved in kerosene or dodecane. In a pulsed column uranium and plutonium enter the organic phase while the fission products and other elements remain in the aqueous raffinate.
In a second pulsed column uranium is separated from plutonium by reduction with excess U4+ added to the aqueous stream. Plutonium is then transferred to the aqueous phase while the mixture of U4+ and U6+ remains in the organic phase. It is then stripped from the organic solvent with dilute nitric acid.
The plutonium nitrate is concentrated by evaporation then subject to an oxalate precipitation process followed by calcination to produce PuO2 in powder form. The uranium nitrate is concentrated by evaporation and calcined to produce UO3 in powder form. It is then converted to UO2 product by reduction in hydrogen.

Recycled uranium and plutonium oxides may be combined to make mixed-oxide fuel (MOX). The correct blend (to achieve the desired enrichment) of the two oxides are mechanically milled to form a solid solution of U-PuO2. This is then made into fuel pellets which are sintered and assembled into fuel rods.
The high-level liquid wastes from the first extraction cycles (about 5 m3/tonne of spent fuel) are concentrated by evaporation to some 250-500 litres/tonne of fuel, stored for some years in shielded and cooled tanks, then calcined to produce some 35 kg of solids which are incorporated in borosilicate glass. The glass contains about 11% radioactive oxides. It is poured into heavy stainless steel flasks, a lid is welded on and in UK they are stored in silos ten deep pending disposal.
taken from www.uic.com.au
This ends my magical mystery tour about Uranium. I hope you enjoyed it
Michael Dean, University of Bristol, UK