

 Sea
snakes come from the Family Hydrophiidae, living most or all of their lives in
usually aquatic, marine environments. They are very closely related to the
Family Elapidae which contains "cobra" type snakes, and this is most
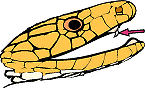
evident in their fang structure. The right image shows the characteristic
small sized fangs towards the front of the mouth, which are used to envenom
their prey. Sea snake fangs are fixed, and unlike many land based snakes,
they do not make lightning fast strikes, instead they tend to hang on and
chew. Mmm.
Sea
snakes come from the Family Hydrophiidae, living most or all of their lives in
usually aquatic, marine environments. They are very closely related to the
Family Elapidae which contains "cobra" type snakes, and this is most
evident in their fang structure. The right image shows the characteristic
small sized fangs towards the front of the mouth, which are used to envenom
their prey. Sea snake fangs are fixed, and unlike many land based snakes,
they do not make lightning fast strikes, instead they tend to hang on and
chew. Mmm.
 Sea
snakes are typically found on tropical shores of the Pacific and Indian oceans
(luckily not the Atlantic). This close proximity to human activity means
that there have been a range of attacks recorded although the species as a whole
is not aggressive and will usually shy away. These snakes have evolved
special salivary glands that produce venom which functions to immobilise and
digest prey. Their venom is one of the most deadly in the world,
containing a lethal cocktail of proteins and neurotoxins. Sea snake venom
has been found to be 2-10 times as venomous as any terrestrial snake making them
extremely deadly, but their docile nature and relatively extreme environment
make them less dangerous than their land dwelling relatives.
Sea
snakes are typically found on tropical shores of the Pacific and Indian oceans
(luckily not the Atlantic). This close proximity to human activity means
that there have been a range of attacks recorded although the species as a whole
is not aggressive and will usually shy away. These snakes have evolved
special salivary glands that produce venom which functions to immobilise and
digest prey. Their venom is one of the most deadly in the world,
containing a lethal cocktail of proteins and neurotoxins. Sea snake venom
has been found to be 2-10 times as venomous as any terrestrial snake making them
extremely deadly, but their docile nature and relatively extreme environment
make them less dangerous than their land dwelling relatives.
The venom contains a series of proteins and neurotoxins.
|
Proteins |
Neurotoxins |
|
lecithinase |
erabutoxin a |
|
anticoagulase |
erabutoxin b |
|
hyaluronidase |
erabutoxin c |
The neurotoxins are by far the most active constituent of the venom and work by acting on the acetylcholine receptor. This causes paralysis of skeletal muscle and death results by respiratory arrest. The venom is potent, but only small amounts are usually injected so fatalities are rare. This is coupled with an observed reluctance to deliver venom when they bite.
The most active component of the venom is erabutoxin b, a short chain protein that consists of 62 amino acids. The primary structure can be illustrated as follows:
N-terminal-ARG-ILE-CYS-PHE-ASN-GLN-HIS-SER-SER-GLN-
PRO-GLN-THR-THR-LYS-THR-CYS-PRO-SER-GLY-SER-GLU-
SER-CYS-TYR-HIS-LYS-GLN-TRP-SER-ASP-PHE-ARG-GLY-
THR-ILE-ILE-GLU-ARG-GLY-CYS-GLY-CYS-PRO-THR-VAL
-LYS-PRO-GLY-ILE-LYS-LEU-SER-CYS-CYS-GLU-SER-GLU-
VAL-CYS-ASN-ASN-C-Terminal
Like so many other neurotoxins erabutoxin b contains 4 disulfide bridges, which are known to be incredibly important in the toxicity of the venom. The neurotoxin has an anti-parallel Beta sheet structure containing no alpha helix structures.
Erabutoxin b
The mechanism that erabutoxin b exerts is not yet fully understood but it is believed to act at acetylcholine receptors. Upon envenomation the toxin binds to the nicotinic acetlycholine receptor on the motor end plate blocking it irreversibly. Acetlycholine binds to an unaffected receptor and opens an ion channel, this results in the depolarization of the end plate through the influx of Na+ ions. If the depolarization then causes an action potential a skeletal muscle contraction occurs. But in the presence of erabutoxin b there is a neuromuscular blockade between the phrenic nerve and the diaphragm. The diaphragm is paralysed and death results from respiratory arrest.
The most amazing aspect of this toxin is its biosynthesis, in that it is synthesized extremely quickly. The rate of biosynthesis was experimentally found by injecting labeled isoleucine into the venom glands. This showed the synthesis starting 30 seconds after injection and finishing 1 minute after injection, i.e. between 30 seconds - 1 minute! For more info.