|
Solar cells are photovoltaic devices, which are devices that can convert light into electricity. They are much in demand as they offer an inexhaustible and environmentally benign energy source. Most solar cells are made of amorphous silicon. The problem with this is that the silicon must be of a very |
|
|
high purity and have a near perfect crystal structure. This makes it very expensive to produce. The efficiency of such a cell is also very small, typically converting only 13-18 % of sunlight to electricity. However, low efficiency wouldn’t matter if huge arrays of cells could be produced cheaply. After all, nature’s solar cells, chloroplasts in plants are less than 1 % efficient.
|
|
|
|
Most solar powered
devices rely on the same principle: a photon of sunlight boosts an
electron in the material into a mobile state so that it can be used
to generate electricity. The problem with this simple mechanism is
that the electrons are negatively charged and will leave a positive
charge. These opposite charges attract one another and therefore
will tend to recombine, squandering the absorbed energy as heat or
as re-emitted light. |
Silicon solar cells use an electric field to push the negatively charged electrons and positive charges apart. While chloroplasts adopt a more subtle approach of separate charges by making a distinction between the units that generate the electron and those that transport it away.
Deciding to copy nature’s trick, Michael Gratzel and Brian O’Regan at the Swiss Federal Institute of Technology began research and produced the Grätzel cell in 1991.
The Grätzel cell uses intensely colored organic dye molecules to capture light energy to inject an electron from the dye into a semiconductor such as titanium dioxide (TiO2). This remarkably efficient charge separation reaction initiates current flow and the output of electrical energy by the cell.
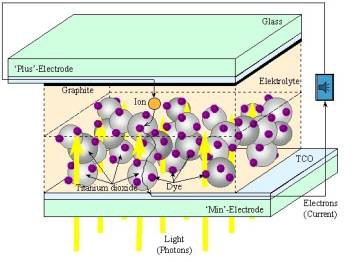
Schematic diagram showing how a Grätzel
cell work. The dye molecules attached onto the semiconductor particles are
sandwiched between two glass electrodes.
How does nanoscience help?
The ideal material used in the cell must have a high surface area for light absorption and charge separation. Nanoparticles, having a comparable surface area to volume ratio, provides for just that. Titanium dioxide nanoparticles are used to make nanoporous thin film supported upon a glass substrate. The material obtained has optical transparency, excellent stability and good electrical conductivity.
The benefit of these novel photoelectrical solar cells is that they can be fabricated from cheap, low purity materials by simple and low cost procedures. Contrary to expectation, some of the new devices also have strikingly high conversion efficiency. The size-tunable bandgaps of the semiconductor nanoparticles, due to size quantisation, also means more efficient solar cells can be produced for photovoltaics (electricity production) and water splitting (hydrogen production) processes.