Electrochemiluminescence
in 1929, Newton Harvey reported "I have recently observed a quite bright luminescence that can be used as a test for active oxygen, formed on anodes during the passage of an electric current. This involves the use of a chemiluminescent compound, aminophthalichydrazid [today; Luminol]".
The high sensitivity (picomlar) of this reaction today (with peroxide) is used in HPLC and immunoassays.
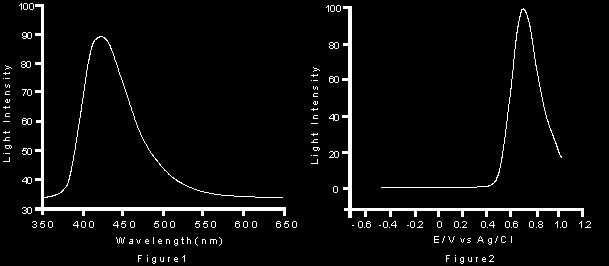
The left hand peak shows peak emission occurs at around 424nm as with normal chemiluminescence (this would be expected). The right hand graph shows the intensity of luminescence with voltage. The first appearance of luminescence follows closely the oxidation potential of luminol to its 3-APA supporting further the mechanisms proposed.
Reaction Mechanisms
These are based on the Liverpool Electrochemiluminescent Group's work.
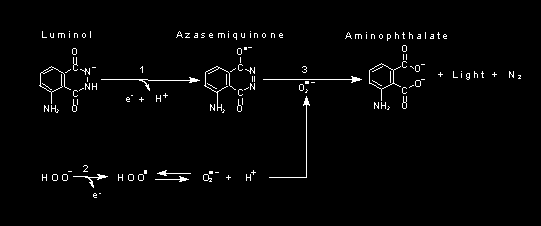
There are two main mechanisms to luminol electrochemiluminescence
- The reaction with oxygen proceeds exactly as in the normal reaction.
- A second reaction occurs with hydrogen peroxide where the hydrogen peroxide and luminol become oxidised to radicals before following the first reaction.
Experimental Suggestions
A simple demonstration of this phenomena can be performed by taking a small amount of my luminol solution and passing a DC current through it. A blue luminescence will appear at one of the lectrodes (think about which one and why) and diffuse out, lingering after removal of current.
This experiment works with copper, iron and nickel electrodes but not with zinc.