|
Potential Energy Surfaces for Diatomic Molecules
|
We saw in section 3.1 that the
potential energy surface of a molecular system is defined as the electronic
potential energy including nuclear repulsion, at a given nuclear configuration
(equation (3.8)). This is found
by solving the electronic Schrödinger equation (equation
(3.6), see also equation (3.4)
for the electronic Hamiltonian operator) for a given nuclear configuration
(inter-nuclear distances and angles).
A potential energy surface is the result of solving equation
(3.6) for many nuclear configurations – giving the electronic
potential energy including nuclear repulsion of the system as a function
of the nuclear coordinates.
In a diatomic molecule AB, there is only one nuclear coordinate; the
inter-atomic distance RAB. In this
case, the potential energy ‘surface’ is more accurately termed
a potential energy curve. It describes the potential energy of the system,
U(R), as the two
atoms are brought closer to, or moved away from, one another (Figure
4.1).
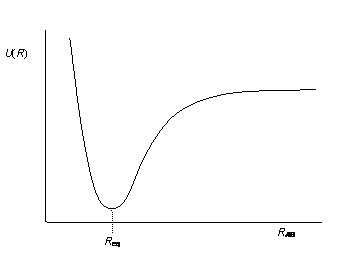 |
| Figure 4.1 - Potential energy curve
for a bound electronic state of a diatomic molecule |
The point at which the curve flattens out at large inter-atomic distances
is termed the dissociation limit, and
represents a state where the molecule is no longer bound, being instead,
two separate atoms.
The dissociation energy, De, is
the vertical distance between the dissociation limit and the base of the
curve, found at the equilibrium bond length, Req.
The following Flash animation (Figure 4.2) illustrates the change in energy
(watch the slider on the left) and inter-atomic distance (watch the atoms
on the right) at different points along the curve:
The curve may not be a bound state – many excited states are unbound,
the typical shape of these curves is exponential decay-like, with no minima
(Figure 4.3, below).
 |
| Figure 4.3 -
Potential energy curve for an unbound electronic state of a diatomic
molecule |
|
| «
Previous | Next » |
|