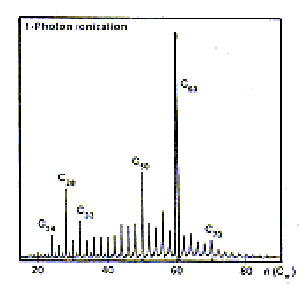
Buckminsterfullerene was first discovered in September 1985, when Richard Smalley, Robert Curl, James Heath and Sean O'Brien from Rice University, Houston worked together with Harold Kroto from the University of Sussex. Kroto was interested in whether large carbon chains could be formed in the extreme conditions of stars' outer atmospheres. Smalley had developed a piece of apparatus that would allow him to blast atoms off the surface of a substance with a high powered laser. Loading the American apparatus with graphite and blasting it with the laser produced the expected long-chained carbon molecules were found. The scientists were surprised to find that there were also small traces of a very stable molecule of mass 720amu (the mass of 12 carbon atoms).

When the experiment was done under the right conditions, the mass spectrum showed a peak at 720amu with an extremely strong signal. The team concentrated on finding out more about this anomaly.
Smalley set about trying to make a model of this atom using paper and sellotape. He discovered that such a structure was not possible using only hexagons, but when he used 20 hexagons and 12 pentagons, he got a spherical shape with 60 vertices. This closed cage structure would account for the stability of the molecule, but it was merely speculative. The new molecule was named buckminsterfullerene in honor of R. Buckminster Fuller, an architect who built a geodesic dome like this at Expo '67 in Montreal.
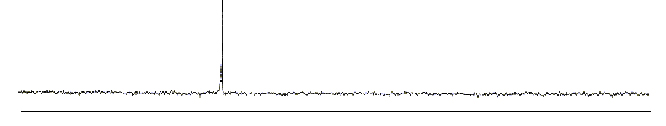
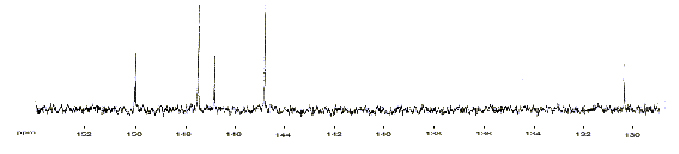
This proposed structure however needed to be proved. The chemists needed larger samples of C60 to prove it's structure, but it could only be produced in minute quantities. A discovery by W Krätschmer and D Huffman some years earlier (some bumps on the UV absorption spectra of carbon soot formed by electrically heating graphite rods) seemed to hint that buckminsterfullerene was being formed here too - in much larger quantities! They could produce soot containing 10% C60 with a small amount of C70. In late 1989 the news of this reached Kroto in Sussex. He managed to reproduce this and in 1990, when dissolving the soot in benzene, he obtained a red solution (this couldn't happen with other known forms of carbon). It had to be C60. He recorded a 13C NMR spectrum of C60, and found it only showed one peak, indicating all carbons are in identical positions, agreeing with the geodisic structure proposed earlier. C70 showed 5 peaks in the ratio 10:10:10:20:20, also supporting the proposed rugby ball shape.