|
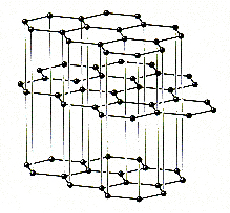
Graphite
|
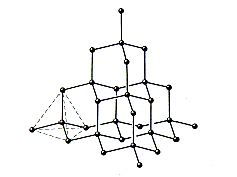
Diamond
|
|
 |
 |
|
|
Fully
delocalised fused benzenoid pi system. Consists of honeycomb layers
of sp2 hybridised C atoms. These layers are 3.35A apart. Graphite is
black and a good conductor due to it's delocalisation. It has lubricating
properties due to the ability of the layers to slide over each other.
|
Sp3
hybridised C atoms form a system of cross-linked cyclohexane chair conformers.
Diamond is colourless and is the hardest substance known to man. This
structure is less stable than graphite and will turn into graphite at
high temperatures or under high-energy radiation.
|
This was thought to be about it until a momentus discovery in 1985 by scientists at the university of Sussex...