Carbon dioxide is transported in the blood in three forms:
![]()
Carbon dioxide is transported in the blood in three forms:
| Approximately 90% as bicarbonate |
| Approximately 5% as carbamino compounds |
| Approximately 5% in physical solution |
|
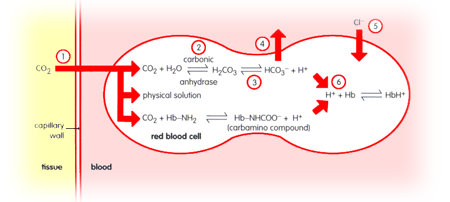
(1)
|
Carbon
dioxide passes from tissue to blood down its partial pressure gradient.
|
|
(2)
|
The
carbon dioxide combines with water to form carbonic acid.
|
|
(3)
|
Carbonic
acid dissociates into protons and bicarbonate ions due to carbonic acid
being a weak acid.
|
|
(4)
|
As
bicarbonate ions are produced a concentration gradient is set up between
the red blood cell and the blood plasma. As a result bicarbonate ions
diffuse out of the cell and into the plasma.
|
|
(5)
|
Bicarbonate
ions exiting the cell results in the red blood cell being positively
charged relative to the blood plasma. Therefore chloride ions enter
the cell from the plasma to maintain electrical neutrality. This exchange
is called the chloride shift.
|
|
(6)
|
Protons
are produced from dissociation of carbonic acid and also of carbamino
compounds. These protons cannot exit the cell due to the redcell membrane
being impermeable to protons, the imidazole groups of haemoglobin therefore
act as a buffer to remove the protons.
|