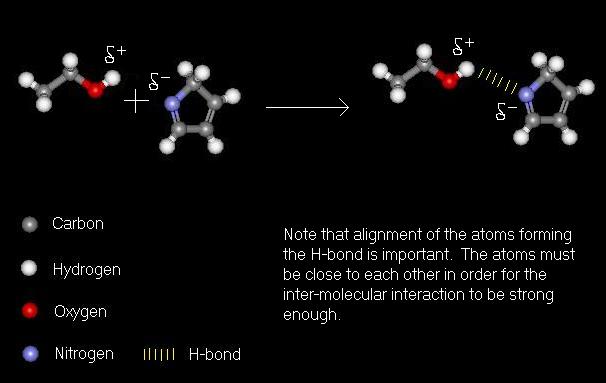
Hydrogen Bonding
Hydrogen bonding, or H-bonding, is a relatively weak inter-molecular interaction. It occurs as a result of electronegativity. When a hydrogen atom forms a bond to a strongly electronegative atom, such as nitrogen or oxygen, the bonds become polarised. This is because the electronegative atom draws a greater portion of the electron density (the bond) towards it, and hence carries a negative charge (although not as much of a charge as an ion). The electronegative atom is said to be d-negative, or d-. As the hydrogen atom has less of the electron share it is said to be d-positive, or d+. If this d+ hydrogen come near to another electronegative atom, a weak interaction is caused by the charges, which holds atoms together. This H-bond has a value of around 10kJ per mole, compared to 100kJ per mole for a typical covalent bond. However, it is by far the strongest of the inter-molecular interactions.
|
Formation of a hydrogen bond
|
 |
Other inter-molecular interactions include Van-der-Waals interactions, where weak attractions form between atoms due to the random movement of electrons in the bond, permanent dipole-permanent-dipole interactions, which is similar to H-bonding, though involving a weaker interaction between a d-negative atom and a non-hydrogen d-positive atom, along with permanent dipole-induced dipole interactions, where a d-negative or d-positive atom induces a dipole on another atom due to coulombic repulsion.