One of the great things about the Big Bang model of universal creation, is that it explains the chemical composition of the Universe very well.
With the appearance of the four fundamental forces, (gravity, electromagnetism and the strong and weak nuclear forces), quarks, then atomic particles and their antimatter partners, appeared. Today, most of the matter in the universe is made up of hydrogen and helium, with only very small proportions of heavier elements. So how does this link to the Big Bang, and why are the relative abundances of the elements explained?
The shortly after the big bang was very hot and very dense. At temperatures of a few thousand degrees, atoms will lose all there electrons, making fully ionized plasmas, (gaseous mixtures of free electrons and nuclei), but after the big bang temperatures were even higher than this, being several million degrees! At these temperatures nuclei themselves break down to their fundamental particles forming a mixture of free fundamental particles (protons, neutrons and electrons).
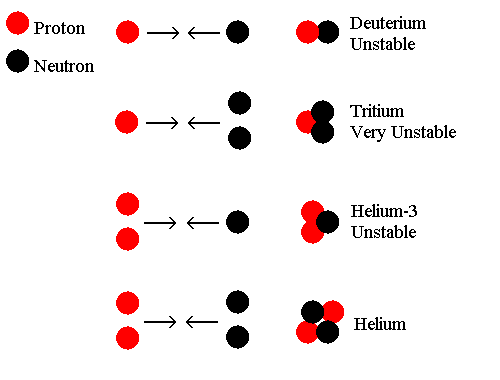
During this period, shortly after the big bang, pressures were also very high and therefore the mixture was very dense. Accordingly, there was a very high collision frequency between these particles, and occasionally these particles would collide to form the nuclei of light elements. This is called Big Bang Nucleosynthesis:-

Clearly the combination of these particles is capable of forming helium, (and as hydrogen nuclei are simply protons anyway, hydrogen does not need to form by combinations of anything) and the unstable helium-3, tritium and deuterium simply break down. But why does the helium not also simply break down again under pressure, and why do heavier elements not form?
In fact, heavier elements do form, but like the helium-3, tritium and deuterium they are formed as unstable isotopes:

Ordinary helium, (helium-4) is the most stable isotope formed in this mixture of fundamental particles and a great deal of it is formed. Of course under very high pressures and temperatures, such as those immediately following the Big Bang, many helium nuclei do break down again, but it must be remembered that at the time, the Universe was rapidly expanding and cooling.
As the temperature and density fell, collisions between particles became less violent, and less frequent. For about one minute, roughly three minutes after the Big Bang, there was a "window of opportunity" when protons and neutrons could collide hard enough to stick together and form light nuclei, yet not suffer so many subsequent collisions that the nuclei would be destroyed. During this window of opportunity all the helium in the Universe was created.
Almost all nuclei heavier than hydrogen formed would be unstable and break down. The only other elements formed to even a small degree were deuterium, and lithium-7, which though stable requires the collision of several particles simultaneously, and it therefore very unlikely.
The exact ratios of hydrogen, helium, lithium and deuterium created during the period of Big Bang nucleosynthesis would have been very sensitive to the density of the mixture of fundamental particles from which they were made. Because the mixture was not entirely uniform in density, different amounts of each were created in different areas of the compacted early universe. As an average though, approximately twelve protons (hydrogen nuclei) were left for each helium nucleus produced.
Of course, this process simply forms nuclei, leaving the ionized plasma described earlier. Free gaseous nuclei mixed with free electrons. The temperature did not drop low enough (below 3000K) for recombination, the gathering of electrons by nuclei, to occur for around 100,000 years!
So, after four minutes, the Universe had made the first basic nuclei, and after 100 millenia it had created the first atoms. But where did everything else come from? How did chemistry create the World, and Universe we see around us today?