

The two types of bonding between the tetrahedra are corner sharing and bridging. In corner sharing the corner linked tetrahedral Si-O-Si bond angle can vary between 120 and 180 degrees.
In bridging the the Si atoms are displaced from the centre of the tetrahedra away from the bridging oxygens due to the repulsive power of two Si atoms.
The silicate in Talc is formed in infinite sheets.
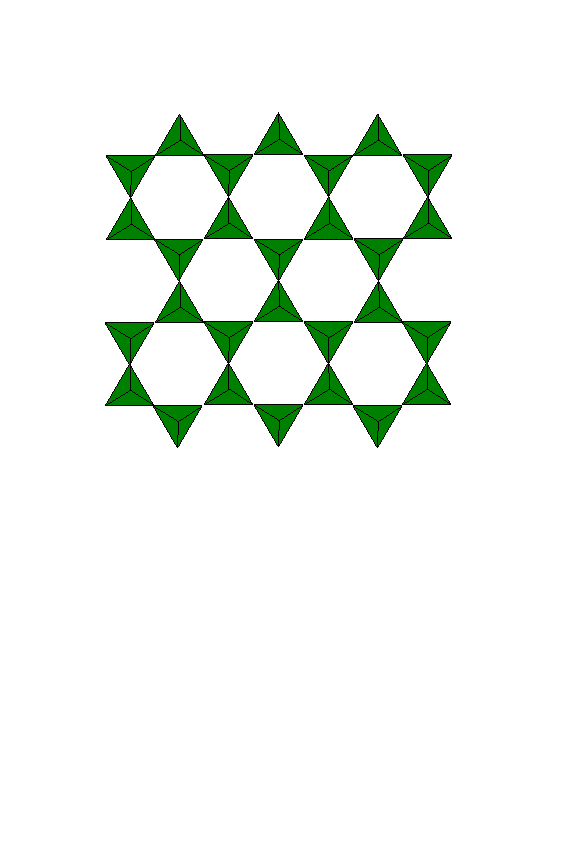
An infinite sheet is composed of tetrahedra each with three bridging and one non-bridging oxygen. This gives a ratio of silicon to oxygen of 1:2.5 and a net charge of (Si2O5)n2n-. The unbridged oxygens all point in the same direction (i.e. all out of the page) and connect the tetrahedral sheet to the octahedral sheet (i.e. brucite layer).
 |