Mechanism of supramolecular chemistry
The
basis of supramolecular chemistry is the binding or complexation event, which is
the action of the host molecule binding with a guest
molecule to form a host-guest complex or supramolecule.
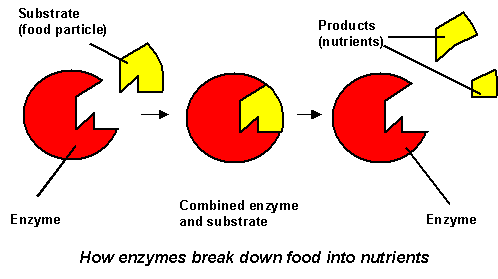
Supramolecular chemistry is characterized by the specificity and selectivity of its reactions. The term used to describe this is molecular recognition, as if the reactions will only happen if the molecule recognizes each other. Therefore, a supramolecular interaction can only happen when the host and guest complement each other. (e.g. hydrogen bond donor/acceptor, Lewis acids/base, hardness or softness etc.) An analogy to the mechanism is the lock and key scenario coined by Emil Fischer in 1894. The host molecule serves as the lock and the guest molecule serves as the key. Only when the key matches the lock will the lock open.

Figure
3 Example if a lock key scenario in biological systems