|
|
|
|||||||
World of Colour
|
The physical measurement of colour | |
Measuring Concentrations using absorbance, | |
Transition Metal Solutions, |
Rhodopsin and the eye
Basics behind Dyes and Pigments
Phosphorescence and Fluorescence
A Thermochromic example
Colour Therapy
Detection using Optical Sensors
Symbols, Glossary, Disclaimer, Credits, References, feedback..
Action 3: Book turning. This image is taken from gifs (ref. 14) and is copyright restricted according to the source given (i.e. it is not the authors' own work).
Visible Region (700-400nm)
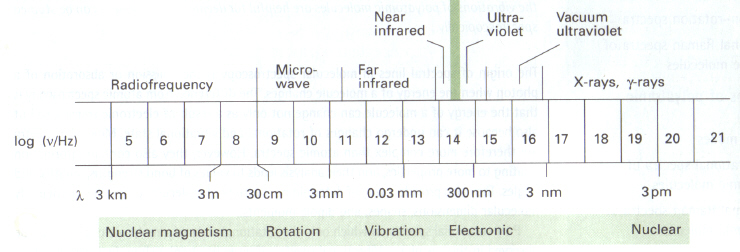
Figure 1: The electromagnetic spectrum. This image is taken from Atkins (ref. 3) and is copyright restricted according to the source given (i.e. it is not the authors' own work).
"White" light contains the entire range of wavelengths within the visible region of the spectrum and if shone through a prism splits into a rainbow of respective colours:

Action 4: Light splitting by a prism. This image is taken from gifs (ref. 14) and is copyright restricted according to the source given (i.e. it is not the authors' own work).
An object appears coloured as it absorbs, scatters or emits electromagnetic radiation within the visible region.
Absorption A photon of light will be absorbed if its energy corresponds to the gap between two energy states. The main examples within this group are excitation of a ground state electron (in the HOMO) into the higher energy excited electronic configuration (or LUMO). The molecules considered can be simple metal ions or conjugated organic molecules (e.g. β-carotene).
Scattering This is the process by which the path of light changes due to multiple refractions and reflections. Scattering centres are often present in opaque objects.
Emission This is when an excited electronic state relaxes back into the ground state thereby releasing a photon of energy equivalent to the energy separation of the two levels involved. The observed colour corresponds to the wavelength of the photon emitted. This is the basis behind both phosphorescence and fluorescence.
