The Chemistry
At the turn of the 19th century it was recognized that acid-catalyzed hydrolysis was the main cause of cellulose depolymerisation, and the source of acidity was due to the hydrolysis of the aluminium sulfate- sizing agents (alum-rosin, which are introduced into the paper during manufacture). The alum-rosin reacts with moisture in the air (i.e. atmospheric hydrolysis) to produce sulfuric acid:
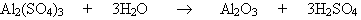
The acid rapidly hydrolyses the glycosidic bonds that hold the cellulose together resulting in a significant reduction in the degree of polymerization, hence molecular mass and paper strength (as well as embrittlement of the pages). Oxidation also causes deterioration, the hydrolysis of the cellulose is prompted by the presence of oxide groups and the presence of transition metals catalyse the oxidation processes, (including the conversion of sulfur oxide to sulfuric acid). Lignin and rosin can combine to form peroxides, which are very powerful oxidation agents and react directly on the different chemical groups of cellulose, breaking it down.