
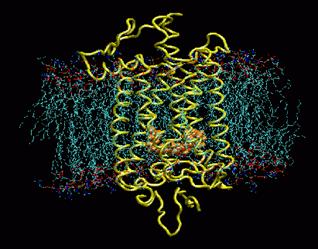
The visual pigment of rods is a deep purple pigment called rhodopsin. Rhodopsin is the a seven trans membrane protein and is from the family of G protein coupled receptors (GPCRs). These trans membrane proteins switch from an inactive to an active form on the binding of a ligand. The activated receptor can trigger an intracellular signal cascade.
Rhodopsin buried in a POPC membrane. Retinal is shown in orange. Image taken from www.ks.uiuc.edu/Research /rhodopsin/ without permission.
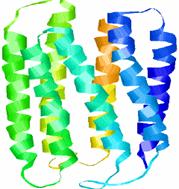
Rhodopsin is arranged in a single layer in the membranes of each of the thousands of discs in the rods outer segment. Rhodopsin forms light through the entire spectrum but it preferentially absorbs green light at a wavelength of 497nm.
Image shows the structure of bacteriorhodopsin. Its structure and function is similar to the protein in the human eye. Image taken from http://instruct1.cit.cornell.edu /courses/biomi290/MEMBRANE/RHODOPSIN.HTML
Rhodopsin is formed in the dark from vitamin A.
Vitamin
A is oxidised to 11-cis
retinal and combined
with scotopsin (the opsin
found
in rods) to form rhodopsin. When rhodopsin
absorbs light, retinal
changes from 11-cis to
all-trans retinal. The retinal-scotopsin complex breaks down allowing
them to separate.
This b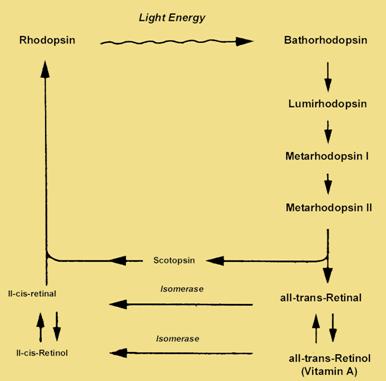 reakdown
is known as the
bleaching of the pigment. The breakdown of
rhodopsin
triggers
a transduction
process involving a
rapid cascade of
intermediates.
Once the all
trans-retinal detaches from the opsin
it is transported to the epithelium by a
protein carrier. Within
the epithelial cells, retinal is
converted to
its 11-cis
isomer in an ATP requiring process. Retinal then heads back to the
photoreceptor cells outer segments to begin the process again.
reakdown
is known as the
bleaching of the pigment. The breakdown of
rhodopsin
triggers
a transduction
process involving a
rapid cascade of
intermediates.
Once the all
trans-retinal detaches from the opsin
it is transported to the epithelium by a
protein carrier. Within
the epithelial cells, retinal is
converted to
its 11-cis
isomer in an ATP requiring process. Retinal then heads back to the
photoreceptor cells outer segments to begin the process again.
Image taken from http://education.vetmed.vt.edu/Curriculum/VM8054/EYE/RHODOPSIN.HTML without permission.
Electronic effects in the rods
In the dark sodium ions leak into the outer segments of the rods. This produces depolarizing potentials that keep calcium channels open in their synaptic endings. This results in continuous neurotransmitter release by the rods at their synapses. When light triggers rhodopsin breakdown the sodium permeability of the outer segment membrane decreases rapidly. This mechanism involves destruction of cyclic GMP (intracellular second messenger which keeps sodium channels open in the dark). The effect of light turns off the sodium entry causing the rod cells to develop a hyperpolarizing receptor potential that inhibits their release of neurotransmitter.