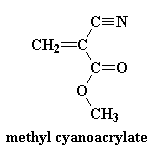
Mechanism of Action
- Polymerisation process activated by initiator in tip
- Polymerises completes with exothermic reaction on contact with moisture to form adhesive film
- Reaches full mechanical strength in 2.5 minutes
- Holding strength is equivalent to a healed tissue after 7 days
- Adhesive film sloughs off within 7 -10 days as skin re-epitheliailses
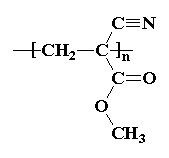
Cyanoacrylates can be synthesized by reacting formaldehyde with alkyl cyanoacetate to obtain a prepolymer that, by heating, is depolymerized into a liquid monomer. The monomer then can be modified by altering the alkoxycarbonyl (-COOR) group of the molecule to obtain compounds of different chain lengths. When the monomer comes into contact with moisture on the skin's surface, it chemically changes into a polymer that binds to the top epithelial layer. This polymer forms a cyanoacrylate bridge, binding the two wound edges together and allowing normal healing to occur below. The conversion from monomer to polymer occurs rapidly, preventing seepage of the adhesive below the wound margins as long as the edges are well apposed. Heat is often generated during the change from monomer to polymer, and this heat may be felt on occasion by patients during application to the skin. Cyanoacrylates have also been shown to have antimicrobial properties.
| Structure of monomer: | Structure of polycyanoacrylate: |
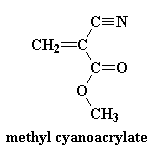
|

|