Box 1:
Zipping up monomers
A major part of
industrial polymer chemistry, not only for adhesives, but also for
plastics, fibres and paints, consists of linking up molecules of
general formula CH2=CXY (vinylic
monomers) to form long chains: CH2
CXYCH2CXY CH2CXY
etc.
When X and Y are
both hydrogen we obtain polyethylene, when X is hydrogen and Y is
chlorine the result is polyvinylchloride (PVC). Vinylic monomers
of importance in reactive adhesives include:
• ethyl cyanoacrylate X = CN, Y = CO2
C2H5
(the basis of superglue);
• methyl methacrylate X = CH3,
Y = CO2CH3
(the basis of Perspex or Plexiglass); and
• the related polyethylene glycol dimethacrylate:
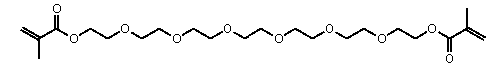
This polymerisation can be
effected by a variety of reactive initiators.
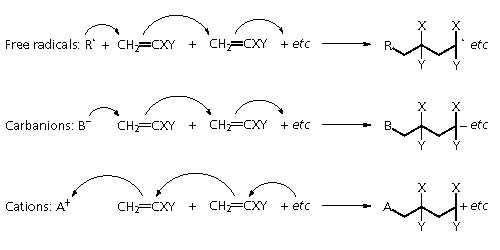
Free radicals can be formed
as required in a number of ways:
• thermally, by the
breakdown of, for example, benzoyl peroxide:
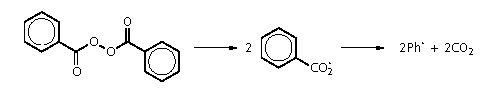
• by a redox reaction
between, for instance, a hydroperoxide and a reducing species:
ROOH + Fe2+
® RO¡P + Fe3+
+ OH¡Â
• photolytically, by the
action of ultraviolet light on a photoinitiator such as benzoin
ether:
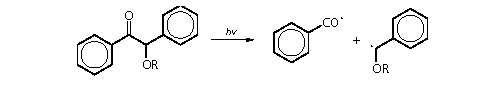
Carbanions are not of
particular importance in adhesives, but cyanoacrylates, with their
two electron withdrawing groups attached to the vinyl group, will
not only react with carbanions, but with virtually any nucleophile,
such as traces of amine on the skin:
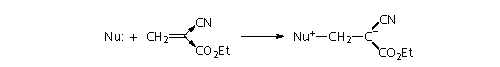
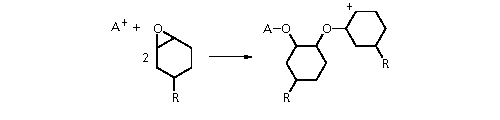
Cations are not used a great
deal with vinyl monomers, but are used with some specialised
epoxies (the epoxy group can be seen as an oxidised vinyl group).
The cations are generated photochemically from species such as Ph3S+SbF
6 ¡Â.
|