Box 2:
Chain extensions
These include the
polycondensations used for making nylon and polyesters. In the
world of adhesives and sealants, the group includes epoxies,
urethanes, silicones and formaldehyde resins. Some typical
reactions - involving both polycondensations and polyadditions -
are shown below.
Epoxies:
The most common epoxy resin used in adhesives is the diglycidyl
ether of bisphenol A (DGEBA). This is typically cured with amine
or mercapto hardeners. Note that the cure reaction with a primary
amine leaves an additional amino hydrogen which can further react
with epoxide groups.
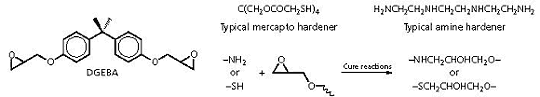
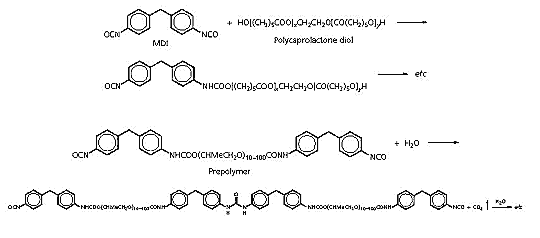
Urethanes:
The two most common reactions are those between a di- or
polyisocyanate, eg diisocyanatodiphenylmethane (MDI) and a polyol
(perhaps a polycaprolactone diol), (a), or between an isocyanate
terminated prepolymer and atmospheric moisture (b).
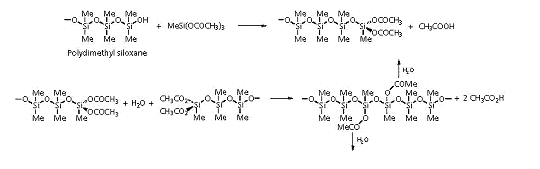
Silicones:
Silicone sealants are based on polydimethyl siloxanes. They
typically contain a crosslinker, methyl triacetoxysilane, and are
cured by atmospheric moisture. The curing reaction generates
ethanoic acid, which accounts for the distinctive smell that you
encounter if you use these products around the house.
Formaldehyde
resins: These are most commonly used in a particular subset
of adhesives: binders (eg for woodchip). They are produced by
reacting methanol with a variety of active hydrogen compounds,
notably phenol, urea and melamine.
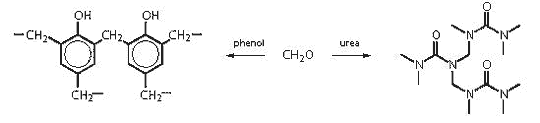
|