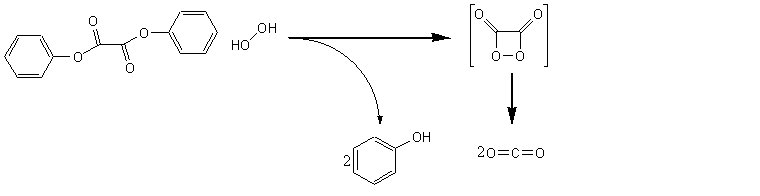
The Chemical Reaction
The chemical reaction is as follows:
The hydrogen peroxide oxidises the oxalic phthalate ester to form phenol and a cyclic intermediate known as 1,2-dioxetane-3,4-dione, which then decomposes to carbon dioxide:

http://www.sas.upenn.edu/~mtc/Lightstick.html
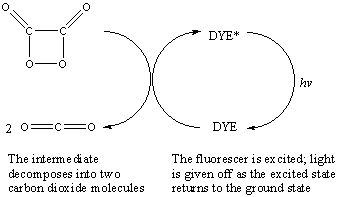
The energy that is released from this decomposition reaction is used to excite the fluorescent dye. As the excited site decays, a photon in the visible spectrum is emitted producing light that we observe as a coloured glow.
http://www.sas.upenn.edu/~mtc/Lightstick.html
The chemistry of light sticks relies on this transfer of chemical energy from a cyclic intermediate that is high in energy to a dye molecule that can release photons in the visible spectrum as it relaxes back to the ground state. The energy barrier is at least 40-70 kcal/mol so this amount of energy must be produced in order for the emitted radiation to be in the visible spectrum. The decomposition of the cyclic intermediate produces this amount of energy so is the most important part of the light stick reaction. Normally, release of the energy would be slow as the product, carbon dioxide, is a small molecule so is unable to accept a large amount of chemical energy. This is overcome by the presence of the dye molecule which receives the energy instead and decays to release photons of visible light.