
Resonance
This an important concept for explaining unexpected properties of compounds such as Benzene. At the time the following was the accepted structure for Benzene.
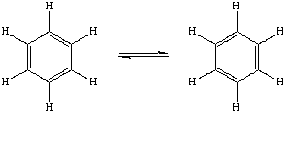
It was proposed by Kekulé that these two structures were in equilibrium with eachother. There were three observations however that this structure did not account for :-
1. The energy of hydrogenation was much higher than expected which suggested Benzene was more stable than predicted.
2. Compounds with double bond normally undergo addition reactions which Benzene does not.
3. X-ray diffraction studies did not agree with this suggestion.
Paulings concept of resonance suggests that structure of benzene does continually change but actually has an intermediate structure somewhere between then two. This is the reason why Benzene is sometimes drawn like this.
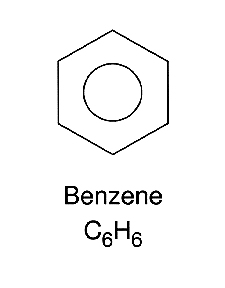
This is drawn like this to show that the π bonds are delocalised over the whole structure which is what explains the three observations above.