A boost for cars (NOS)
|
Dom: You almost had me? You never had me - you never had your car... Granny shiftin' not double clutchin' like you should. You're lucky that hundred shot of NOS didn't blow the welds on the intake! |
|
|
U.K. CERTIFICATE: 15 |
|
|
RUNTIME: 106 mins |
|
|
SYNOPSIS: Races are mostly won by one of the drivers firing nitrous oxide into the intake manifold, on the final stretch. The nitrous oxide systems (NOS) play an important part in the movie, whereby Brian O'Conner (Paul Walker) believes his power NOS is crucial to win a drag race. |
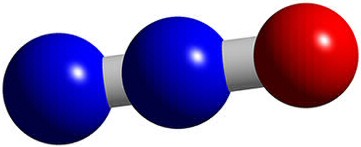
Nitrous Oxide
|
History and Preparation: Joseph Priestley discovered nitrous oxide gas in 1772. Priestley first made N2O by heating ammonium nitrate (NH4NO3) in the presence of iron filings, and then passing NO (the generated gas) through H2O removing any toxic by-products. 2NO + H2O + FeNowadays nitrous oxide can be commonly made in the laboratory by fusing and boiling NH4NO3 to produce steam, N2O, N2, NH4NO3 'fog' and small amounts of very toxic higher oxides of nitrogen (i.e. NO2, NO). NH4NO3 |
Click on the picture above
to interact Resonance Hybrid: Chemical
Name: Dinitrogen oxide |
|
When provided with a sufficient amount of energy (via heating) N2O decomposes to N2 and O2 (exothermic reaction):
|
|