|
|
Arzt:
Do you have any idea
what happens to dynamite in
ninety plus degree heat, huh? Do you know? Any of you? It sweats nitroglycerin. Dynamite
is nitroglycerin stabilised by
clay. Nitroglycerin is the most dangerous
and unstable explosive know to man. Any of you hear about the guy who invented
nitroglycerin? Probably not!...cause he blew is frigging face off. His lab
assistance came into the room, saw that his mentor had detonated, and he said;
"huh, I guess this stuff does work". OK were not going to take more of this stuff
than we need, because nitroglycerin is extremely temperamental, so we don’t…[Waves
the dynamite and blows himself up]. |
|
|
CERTIFICATE: 15 |
||
|
RUNTIME: 44 mins |
||
|
SYNOPSIS: Dr. Leslie Arzt (Daniel Roebuck) accompanies the team venturing to the Black Rock to assist in the retrieval of dynamite to blast open the mysterious hatch. When Jack, Kate, and Locke bring out a crate containing dynamite, an alarmed Arzt explains that, at 90ºF, dynamite sweats nitroglycerin, the most reactive substance known to man. After Arzt wraps a stick of dynamite in Kate's wet shirt, he waves the dynamite (by accident), which explodes in his hands, killing him instantly. |
||
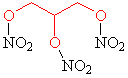
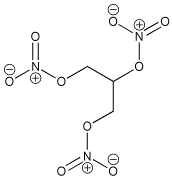
Nitroglycerin
| History: The Italian chemist Ascanio Sobrero first made nitroglycerin in 1847, by adding glycerol to a mixture of concentrated nitric and sulfuric acids. Sobrero’s face was badly scarred due to an explosion in the 1840s. Terrified of his discovery he considered nitroglycerin to be too dangerous for practical use as the impure compound was liable to explode without warning. A Nobel Discovery: Swedish scientist and industralist Alfred Nobel studied these problems and worked hard to improve nitroglycerin as a means for blasting rock and thus used as a tool for mining. In the 1860s he discovered that when the compound was combined with silica it could be turned into a paste and kneaded into shapes. This is frequently referred to as dynamite. Discontent: Sobrero was mortified when Nobel began the commercial exploitation of nitroglycerin and due to the success of dynamite; the Italian felt he had been subject to an injustice. Although Nobel openly cited Sobrero as the inventor of nitroglycerin, Sobrero quoted: "When I think of all the victims killed during nitroglycerin explosions, and the terrible havoc that has been wreaked, which in all probability will continue to occur in the future, I am almost ashamed to admit to be its discoverer".
|
Click on the picture above
to interact
Chemical
Name: Nitroglycerin (or nitroglycerine, trinitroglycerin,
glyceryl trinitrate) |
Instability and Explosive Nature
|
As nitroglycerin contains oxygen, nitrogen and carbon, an explosion is essentially rapid combustion - the three nitrate groups (powerful oxidizing agents) are bound directly to a hydrocarbon fragment (a fuel). Hence releasing a large amount of energy (exothermic reaction) because the atoms rearrange to form new molecules with strong, stable (multiple) bonds, such as N2 and CO.
Pure nitroglycerin is a contact explosive (physical shock may result in an explosion), which degrades over time, producing even more unstable chemical forms. As a result nitroglycerin is highly dangerous to transport or handle. The undiluted form it is one of the most powerful high explosives, analogous to military explosives RDX and PETN, along with the plastic explosive C-4.
One advantage that nitroglycerin possesses in contrast to other high explosives, such as TNT, is that no solid forms of carbon (soot or smoke) are produced once the material is detonated. Therefore nitroglycerin can be used to create 'smokeless powders', which is of a great advantage to the armed forces whose field of vision is not obscured by clouds of billowing smoke during a battle. |
Watch this short clip demonstrating the explosive power of nitroglycerin. Click on the icon below: |
| Season 4; Episode 4; 10:00-11:00 a.m. |
Audrey: Dad, dad, calm down. Are you all right? |
|
|
CERTIFICATE: 15 |
||
|
RUNTIME: 44 mins |
||
|
SYNOPSIS: Secretary of Defense James Heller (William Devane), and his daughter Audrey Raines (Kim Raver), are kidnapped by terrorists and being held captive in a warehouse. |
||
|
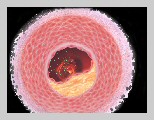
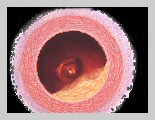
In pharmaceutical form, nitroglycerin (sometimes called glyceryl trinitrate, probably to prevent alarming patients) is used as a heart medication, which is unlike its unstable counterpart, as it cannot be rendered even slightly explosive. It is used as a medicine for angina pectoris in tablets, ointment, solution for intravenous injection, transdermal patches (Transderm Nitro®, Nitro-Dur®), or sprays administered under the tongue (Nitrolingual Pump Spray®). Angina occurs when the coronary arteries become constricted (ischemia) and are incapable of carrying sufficient oxygen to the heart muscle, resulting in suffocating chest pain. The principal action of prescribed nitroglycerin is vasodilation – which is widening (relaxing) of the blood vessels. Nitroglycerin is converted to nitric oxide (a natural vasodilator) within the body, which in turn causes the coronary artery (blood vessels surrounding the heart) to dilate, increasing blood flow and thereby improving the oxygen supply to the heart. As a result chest pains subside as the blood pressure decreases and the heart rate increases. Although nitroglycerin only controls chest pains, it does not cure the condition (i.e. it cannot stop an attack that has already started). Also it may lose effectiveness over long periods of use. Nitroglycerin has the following side effects as the body adjusts to the medication:
|
Trade Names: Nitrospan® and Nitrostat®
|