![]()
Carbon Dioxide
The gas we exhale that's both a Greenhouse gas
and a fire extinguisher
![]()
Mike Thompson and Jess Abel
Rugby School, UK
![]()
Molecule of the Month May 2012
Also available: JSMol version.
![]()
Carbon DioxideThe gas we exhale that's both a Greenhouse gas
|
 |
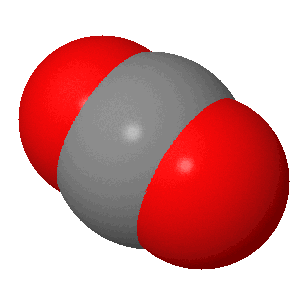
 Carbon dioxide is a simple covalent molecule that most people have heard about, as it is often in the news due to its role in global warming. Carbon dioxide has the formula CO2 and at the centre of this linear molecule is a carbon atom joined by two pairs of double-bonds to the oxygen atoms, i.e O=C=O. At room temperature carbon dioxide is a colourless gas which has a slightly sweet smell. Carbon dioxide is a linear molecule with a bond angle of 180°. This gas was 'discovered' by Scottish scientist (physicist and chemist) Joseph Black (right).
Carbon dioxide is a simple covalent molecule that most people have heard about, as it is often in the news due to its role in global warming. Carbon dioxide has the formula CO2 and at the centre of this linear molecule is a carbon atom joined by two pairs of double-bonds to the oxygen atoms, i.e O=C=O. At room temperature carbon dioxide is a colourless gas which has a slightly sweet smell. Carbon dioxide is a linear molecule with a bond angle of 180°. This gas was 'discovered' by Scottish scientist (physicist and chemist) Joseph Black (right).
Solid carbon dioxide is known by chemists as cardice and by everyone else as dry ice. It is an unusual solid as it sublimes (turning from solid to gas without going through the liquid state). Solid carbon dioxide has weak forces (van der Waals) between molecules which holds them together. This is why it has a very low melting point 217 K (-56°C) at 5.2 atmospheres. Carbon dioxide is non-polar as its dipoles cancel, and this contrasts with the polar molecule sulfur dioxide (SO2).
 Carbon dioxide fire extinguishers contain highly pressurized carbon dioxide which is a non-flammable inert gas, which acts as a smothering material. They work by displacing the air (oxygen) from the area surrounding the fire. Once this part of the fire triangle has been removed the fire is extinguished. This type of fire extinguisher has the added advantage of also cooling the fuel. Carbon dioxide extinguishers are suitable for class B fires (flammable liquids and gases). These extinguishers have the advantage of not leaving a residue after they have been used (discharged). They are suitable for electrical fires in offices containing computers, televisions and photocopiers.
Carbon dioxide fire extinguishers contain highly pressurized carbon dioxide which is a non-flammable inert gas, which acts as a smothering material. They work by displacing the air (oxygen) from the area surrounding the fire. Once this part of the fire triangle has been removed the fire is extinguished. This type of fire extinguisher has the added advantage of also cooling the fuel. Carbon dioxide extinguishers are suitable for class B fires (flammable liquids and gases). These extinguishers have the advantage of not leaving a residue after they have been used (discharged). They are suitable for electrical fires in offices containing computers, televisions and photocopiers.
In the unlikely event that you are faced with a magnesium fire it is not advisable to use a carbon dioxide fire extinguisher because it actually reacts with magnesium.
2 Mg (s) + CO2 (g) ![]() 2 MgO (s) + 2C (s)
2 MgO (s) + 2C (s)
Carbon dioxide will burn on reaction with magnesium and the result is the formation of magnesium oxide and carbon as soot. There are some superb films of the reaction online using blocks of dry ice (solid carbon dioxide). Carbon dioxide is a solid below -78°C. This is why dry ice should not be handled as it causes burns by freezing the skin.
Carbon dioxide is an acidic oxide (a typical property of the majority of non-metal oxides) and reacts with sodium hydroxide to form a salt and water. Next time you are in the laboratory check the top of the sodium hydroxide bottles. If you see a white crust around the stoppers inform the technicians who need to supply fresh solutions. This simple chemistry has been exploited in craft such as submarines and space shuttles where the build up of carbon dioxide from respiration needs tackling.
CO2 (g) + 2 NaOH (aq) ![]() Na2CO3 (aq) + H2O (l)
Na2CO3 (aq) + H2O (l)
The thermal decomposition of lithium carbonate and the following Group 2 carbonates leads to the production of carbon dioxide gas and the metal oxide. Interestingly, beryllium carbonate is unstable under standard conditions (298 K, 100 kPa) and the subsequent Group 1 carbonates are thermally stable.
The trend is that on descending Group 2 their carbonates become more difficult to decompose thermally as reflected by the increasing temperatures. These differences can be explained in terms of charge-density differences for the cations (M2+).
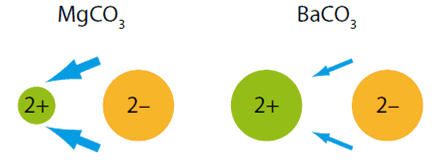
Magnesium's smaller 2+ ion (72 pm) polarises the carbonate 2- ion (135 pm) more than the larger barium 2+ ion (100 pm) because it has greater attractions for its electrons. The size of the 'electron drift' is indicated by the size of the blue arrows.
Diamond is an allotrope of carbon which was first confirmed in a very expensive experiment performed by the English chemist Smithson Tennant who was the first to burn a diamond in air (~600-800°C) producing carbon dioxide.
Carbon dioxide as a non-metallic oxide is acidic. Carbon dioxide reacts with water in a reversible reaction to form carbonic acid (H2CO3).
CO2 (g) + H2O (l) ![]() H2CO3 (aq)
H2CO3 (aq)
The acid dissociation constant for carbonic acid Ka = 4.45 x 10-7 mol dm-3. This indicates that it is a much weaker acid than ethanoic acid which has a Ka = 1.74 x 10-5 mol dm-3. The equilibrium between carbon dioxide and water helps buffer the blood.
CO2 (aq) + H2O (l) ![]() HCO3- (aq) + H+ (aq)
HCO3- (aq) + H+ (aq)
For those doing A-level or Pre-U chemistry, can you show that the pH of blood is 7.35 if the concentration of hydrogen carbonate is 1.3 x 10-2 mol dm-3 and the concentration of carbon dioxide is 1.3 x 10-3 mol dm-3? Answer on page 4 of this link. The carbonic acid present in the equilibrium can neutralize hydroxide ions, which would increase the pH of the blood when added. The bicarbonate ion (HCO3-) can neutralize hydrogen ions (protons), which would cause a decrease in the pH of the blood when added. Both increasing and decreasing pH is life threatening.
The density of carbon dioxide is 1.53 g/cm3 at 21°C. The fact carbon dioxide is heavier than air makes it particularly dangerous when large volumes are released from volcanoes. In 1986 a Cameroon volcano erupted suffocating 1700 villagers with some fortunate survivors asleep on the top bunk bed. When carbon dioxide gas is prepared it sinks to the bottom of a test tube or gas jar and will extinguish a flame. A very familiar test for carbon dioxide is its reaction with a colourless solution of calcium hydroxide which still retains its historical name lime water. A simple experiment is shown and the evolved carbon dioxide often described as effervescence (fizzing) will move from the left hand test tube into the right hand test tube.
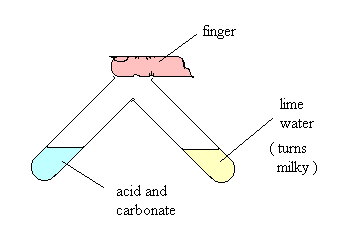
CO2 (g) + Ca(OH)2 (aq) ![]() CaCO3 (s) + H2O (l)
CaCO3 (s) + H2O (l)
Lime, the fruit, tastes sour because it contains acids, whereas limewater (calcium hydroxide) is most definitely an alkali as it contains hydroxide (OH-) ions.
The concentration of carbon dioxide in air is constantly being monitored by thousands of scientists across the globe. As a greenhouse gas changes in carbon dioxide levels contribute to increases in the earth's temperature; the IPCC has concluded that carbon dioxide increases due to fossil fuel burning and deforestation have contributed to most of the global warming over the past 50 years. Carbon dioxide concentrations have also changed throughout Earth history, and geologists study its sources and sinks over a range of timescales.
The story of carbon dioxide brings in some of the most important names in the history of chemistry and its early development from alchemy to the central science. It has long been known that 'fixing air', as carbon dioxide used to be known, could be made by adding an acid to the sedimentary rock, chalk.
CaCO3 (s) + H2SO4 (aq) ![]() CaSO4 (aq) + H2O (l) + CO2 (g)
CaSO4 (aq) + H2O (l) + CO2 (g)
In 1772, Priestley published a paper entitled "Impregnating Water with Fixed Air" in which he described dripping "oil of vitriol" now called sulfuric acid (H2SO4) onto chalk to produce carbon dioxide gas. The gas was dissolved in water by Priestley to make a refreshing drink for his friends. This was the beginning of the first fizzy drink, which is an industry now worth billions of dollars a year to major corporations, and one significant contributor to the obesity and dental caries epidemics in the western world.
In 1822 carbonated water reached Kolkata (formerly Calcutta) in India. Nearly two hundred years later Rugby School has strong links with a school in Kolkata called Future Hope set up by the Old Rugbean philanthropist Tim Grandage. Today carbonated water is made by passing pressurized carbon dioxide through water. The added pressure increases the solubility allowing more carbon dioxide to dissolve than would be possible under standard conditions. When the bottle is opened, the release in pressure allows the gas to come out of the solution, forming the characteristic bubbles. A fun way to rapidly release the gas is to add Mentos to the drink.
The graph below shows how carbon dioxide levels have risen in the atmosphere over recent decades.

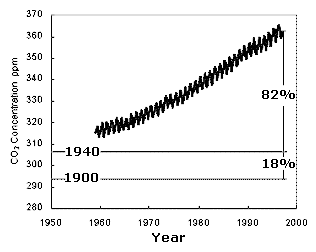
The intra-yearly variations can be attributed to the season, with photosynthesis drawing CO2 out of the atmosphere in the Spring and respiration releasing it back in the autumn. (The northern and southern hemisphere seasons not cancel out, because the former has much greater land mass and therefore terrestrial plant mass!). The plot also shows the dramatic increase in CO2 in the atmosphere over the past century, driven primarily by the combustion of fossil fuels and changes in land use. Click here to get an updated value for atmospheric carbon dioxide in parts per million (ppm). 0.034% = 340 ppm.
At the simplest level, photosynthesis can be represented as the equation which is the reverse of aerobic respiration. Photosynthesis takes place in green plants and fixes carbon dioxide into sugar molecules, as mentioned in the MOTM on glucose.
Photosynthesis: 6 CO2 (g) + 6 H2O (l) ![]() C6H12O6 (aq) + O2 (g)
C6H12O6 (aq) + O2 (g)
Gardeners have exploited this equation by increasing the concentration of carbon dioxide in greenhouses to improve yields. This can be as simple as lighting candles which are solid hydrocarbons (waxes) which when combusted produce carbon dioxide and water.
2 C18H38 (s) + 37 O2 (g) ![]() 36 CO2 (g) + 38 H2O (l)
36 CO2 (g) + 38 H2O (l)
The Carbon cycle is the mechanism by which the carbon dioxide levels are maintained in the atmosphere. Carbon dioxide is central to this important cycle.
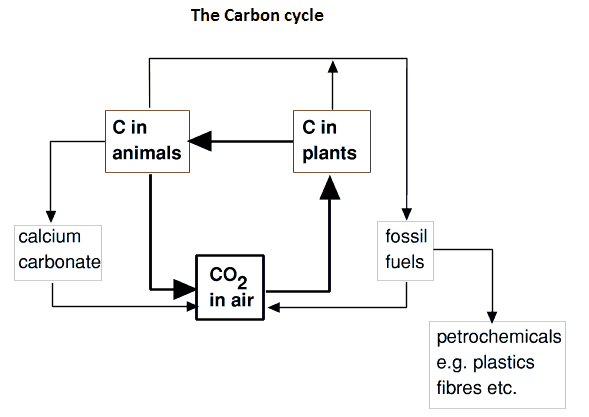
A new technology called carbon capture and storage (CCS) will no doubt be added to future carbon cycles as it is a way to 'remove' carbon in the form of carbon dioxide from the air. Recent news has expressed concern about changes in pH and temperature of sea water and the impact this has on sensitive coral reefs around the world. Scientists often produce some of their best work when they mimic nature and this is what a company in the USA is achieving by mimicking corals by turning carbon dioxide into cement.
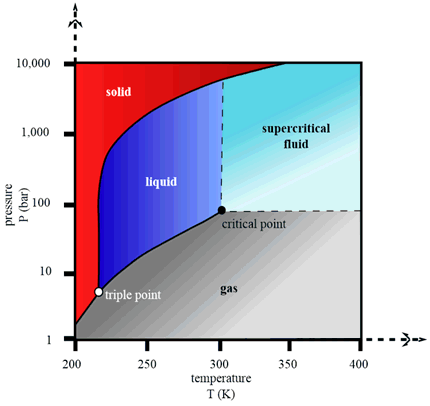
There is strong evidence that the Earth's temperature has warmed by slightly over 1°C since 1880. This increase coincides with and has been attributed to rising atmospheric CO2 levels. These CO2 levels (~415 ppm) are higher than they have been for about 3 million years, since the Pliocene. Carbon capture and storage (CCS) is a new technology that can prevent all the CO2 being emitted primarily from pulverised coal combustion sites, into the atmosphere. Coal accounts for 40% of the world's carbon emissions. Instead, CO2 can be 'captured' and compressed to a supercritical state (10 MPa) before being transported and stored from harm's way. Three main carbon capture methods are; post-combustion, pre-combustion and oxy-fuel combustion.
Post-combustion carbon capture occurs when CO2 is extracted from flue gases after the fossil fuel is burned. Flue gases contain between 15 to 20% CO2. The flue gases are passed through a solvent filter, often an amine, e.g. monoethanolamine (H2NCH2CH2OH) or methyldiethanolamine (CH3N(C2H4OH)2), which can both absorb carbon dioxide. The amine solvent is then passed through a regenerator, which requires 80% of the total energy input for the process. Here, a change in pressure, and or, temperature is applied, releasing water vapour and leaving behind a concentrated stream of CO2. The hydrate crystals formed from these flue gases hold a CO2 concentration of 55-57%. This solvent can then be recycled back into the system and reused. Interestingly, the apparatus needed for this method can be retrofitted to existing power plants.
Pre-combustion carbon capture occurs before the fossil fuel is burned. This process is known as Integrated Gasification Combined Cycle (IGCC). The pulverised coal is first heated to between 537°C and 1426°C in pure oxygen in a 'gasifier'. This leads to the formation of synthesis gas (syngas) which is a mixture of carbon monoxide and hydrogen. CO is then passed through a shift reactor where steam is added, transforming CO into highly concentrated CO2 and hydrogen. Finally, these gases are fed into a flask where they naturally begin to rise. An amine solution is then released into the flask and binds with the CO2. With a newly increased density, this mixture falls to the bottom of the flask and is tapped off. This is now heated to 120°C in a new flask separating the mixture. CO2 rises to the top for collection and the amine falls to the bottom for re-use. The remaining hydrogen can be used as a 'clean fuel' for heat, power and, potentially, as a transport fuel. This process can prevent approximately 90% of carbon emissions from entering the atmosphere. At the time of writing there are one hundred and sixty IGCC plants and another thirty-five IGCC plants in planning.
The third carbon-capture process is oxy-fuel combustion. Here fossil fuel is burnt in a mixture of pure oxygen and recycled flue gas, which regulates the combustion temperature and cools down the reaction from 1550°C to 800°C. This results in more complete combustion, forming 90% CO2 and water vapor. First, sulfur impurities are removed by adding limestone solution, which reacts with SO2 to form gypsum (used in construction industry). Then the resulting mixture is cooled to allow the water vapor to condense and be tapped off. CO2 is now compressed at 70 atm and transported to a storage unit. Before storage, captured carbon, in the form of CO2, must be transported. The most common method of transportation is via pipelines typically made of carbon manganese steel. In the US alone, there are more than 1,500 miles of pipeline used for CO2 transportation. CO2 is mainly driven along pipelines in a dense-phase liquid state by a compressor. In this supercritical phase, CO2 holds the density of a liquid and the viscosity of a gas.
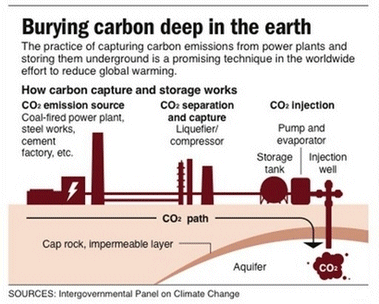
In the final stage of carbon capture, the extracted CO2, must be stored underground or underwater. In 2009, scientists mapped 6,000 m2 that could be used to store 500 years' worth of CO2 emissions from the United States. Underground storage is commonly known as geological sequestration. Typically, CO2 is injected 1,000 m below the ground into layers of porous sedimentary rock under an area described as 'cap rock'. This cap is impermeable and consequently acts as a seal. The pressure underground causes CO2 to behave like a liquid. Declining oil fields and basalt rock formations are the two popular regions for storing CO2. Approximately 30 to 50 million tons of CO2 are injected into declining US oil fields every year. CO2 captured in this way can be used for a process known as Enhanced Oil Recovery (EOR). Underground, at such high temperatures and pressures, injected CO2 will dissolve in oil. This allows recovery of oil buried deep within porous rocks, which would otherwise be impossible to extract. There are currently 70 EOR projects worldwide, mostly in the western United States. If injected into basalt formations, eventually CO2 is expected to react with available metal oxides to form stable carbonates, such as limestone. Their stability in most geological conditions precludes decomposition; and therefore little CO2 is expected to escape back into the atmosphere. A project named 'CO2 sink', started in Berlin in 2004, is investigating the lifetime of CO2 in basalt formations and the stability of its resulting metal carbonates.
Injecting CO2 into the ocean at a depth below 3500 metres is another safe potential method of CO2 storage. The pressure at this depth compresses CO2 into a 'slushy' material, which slowly sinks to the ocean floor. However, this method is as yet largely untested, its impact on deep sea organisms are unknown, and some estimates suggest it is likely to be prohibitively expensive at $40-80 per tonne of CO2 stored.
Carbon dioxide has also found use in removing caffeine from coffee, to produce decaffeinated coffee. There are four main methods which make use of different solvents to extract caffeine from green coffee beans, and they are; water, ethyl ethanoate, liquid (supercritical) carbon dioxide and dichloromethane.
Symbol for decaffeinated coffee (devised by a chemist)
 Baking Soda is the old-fashioned name for sodium hydrogen carbonate (sodium bicarbonate) and has the formula NaHCO3. It is used to make cakes rise in baking as it releases carbon dioxide when it reacts with acidic ingredients. The chemical reactions produce bubbles of carbon dioxide which expand at oven temperatures leading to baked goods expanding (rising) to give light products. The reaction between baking soda and citric acid is shown below. This reaction forms the basis of sherbert and is a classic endothermic reaction.
Baking Soda is the old-fashioned name for sodium hydrogen carbonate (sodium bicarbonate) and has the formula NaHCO3. It is used to make cakes rise in baking as it releases carbon dioxide when it reacts with acidic ingredients. The chemical reactions produce bubbles of carbon dioxide which expand at oven temperatures leading to baked goods expanding (rising) to give light products. The reaction between baking soda and citric acid is shown below. This reaction forms the basis of sherbert and is a classic endothermic reaction.
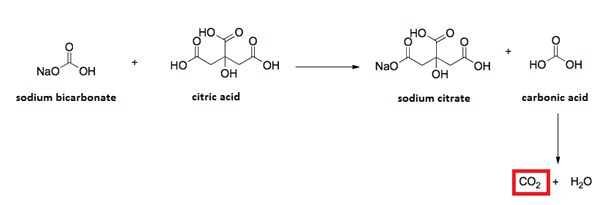
Carbon dioxide is also produced in the anaerobic respiration of sugars by yeast using its enzyme xymase. The by-product ethanol was an ancient way to provide potable drinking water. The reaction also allowed Western style bread to rise as it is baked.
C6H12O6 (aq) ![]() 2 C2H5OH (aq) + 2 CO2 (g)
2 C2H5OH (aq) + 2 CO2 (g)
 Dry Ice in show-business
Dry Ice in show-businessDry ice is frequently used to produce smoke-like clouds in theatres and nightclubs. Dry ice is very cold at -78°C and when it sublimes, because of its density, it stays low on the stage. It soon cools the air it diffuses through and this causes the water vapour to condense into tiny droplets which look like clouds. Dry ice finds use in school Physics laboratories in cloud chambers, cooling thermistors and metal wire resistors, and is used in experiments to confirm the Gas Laws. A colourful experiment to try out is to drop dry ice into a large measuring cylinder which contains water and universal indicator. A horror-film-like effect takes place as the excess carbon dioxide spills over the top of the cylinder and the indicator turns from green to yellow and then orange as carbonic acid is produced. Dry ice lasts a couple of days when stored in a thick-wall polystyrene (Styrofoam) box. It is possible to make dry ice from a cylinder of carbon dioxide if you have the correct dry-ice attachment, sometimes called Snowpacks or Jetfreezers with an explanation of how to use them in section 13.3.1 of the Laboratory Handbook produced by CLEAPSS. Alternately, dry-ice pellets can be obtained from universities or local suppliers, and for those having difficulty getting dry ice there are plenty of good experiments on offer on the internet which give ideas of the demonstration potential of this simple molecule.
 Carbon Dioxide detectors
Carbon Dioxide detectors CO2 detectors are being used to detect dangerous levels of CO2 in laboratories, breweries and cellars. This is important because levels of 1.5% CO2 can cause headaches and hyperventilation, and levels exceeding 10% CO2 levels can lead to death. Crowcon is a leading UK gas detection company that manufactures portable and fixed CO2 monitors within enclosed area.
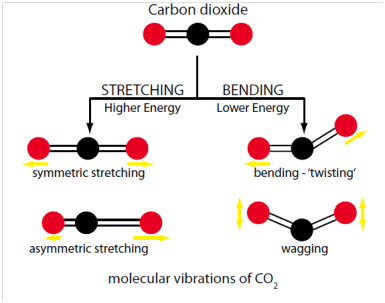
Carbon dioxide levels are detected using an infra-red detector. For ease of use the sensor continuously measures CO2 levels and displays the reading on an easy-to-read backlit display. Units also provide a loud audio alarm. The detectors are manufactured by Crowcon, one of the UK's leading gas detection companies. The infra-red vibration modes for carbon dioxide are show. To be infra-red active a dipole is needed in this molecule which occurs when vibrations lead to asymmetry in the molecule, so dipoles do not cancel.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.14399246]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.14399246]