![]()
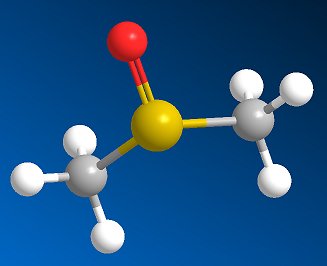 |
Dimethylsulfoxide(DMSO)The smelly solvent that may |
 |
![]()
Paul May
University of Bristol
![]()
Molecule of the Month January 2012
Also available: JSMol version.
![]()
Yes, and no. It is a good solvent which is frequently used for reactions involving salts, especially nucleophilic substitutions. But it has the unusual property that it can pass through mebranes and rubber gloves quite easily, and penetrate the skin. After contact with it on the skin, some people find that DMSO is secreted out onto the surface of the tongue causing a garlic-like taste in the mouth, and garlic breath!
Well, actually, that property is quite useful, because DMSO can dissolve certain useful medicines and transport them through the skin without the need for an injection. DMSO is predominantly used as a localised painkiller, as an anti-inflammatory, and an antioxidant. It is frequently mixed with antifungal medications, enabling them to penetrate the skin, and also toenails and fingernails.
 Who discovered this?
Who discovered this?DMSO is a by-product of kraft pulping, which is the conversion of wood into wood pulp consisting of almost pure cellulose fibres. Wood-chips are treated with a mixture of sodium hydroxide and sodium sulfide (known as white liquor), that break the bonds that link lignin to cellulose. Oxidation of dimethyl sulfide with oxygen or nitrogen dioxide gives DMSO. DMSO was first synthesized in 1866 by the Russian scientist Alexander Zaytsev (photo, right).
But the history of DMSO as a pharmaceutical began in 1961, when Dr. Stanley Jacob was head of the organ transplant program at Oregon Health Sciences University. While investigating the potential for DMSO as a preservative for organs, he discovered that it penetrated the skin quickly and deeply without damaging it, and became intrigued.
|
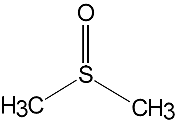 |
DMSO is a clear, colourless, hygroscopic liquid. It is a dipolar aprotic solvent, miscible with water and soluble in many polar organic solvents such as alcohols, ester, ketones and chlorinated solvents. It will dissolve many inorganic salts.
Its ability to penetrate the skin is due to the fact that the molecule is highly polar, but also because it has two methyl groups which interact strongly with lipids in the skin. One particular danger associated with DMSO is that although not considered toxic itself, it is highly effective at transferring other (potentially toxic) substances into the body via skin contact. For example skin contact with DMSO and a cyanide salt would pose a high risk of cyanide poisoning. DMSO will dissolve and penetrate ordinary rubber gloves, so alternative materials should be used such as butyl rubber or blue nitrile.
Although it is non-toxic for short exposures, other safety concerns associated with DMSO are that it can be irritant and harmful at higher dosages. Prolonged exposure can lead to dermatitis, and possibly to liver and kidney damage. It can produce explosive reactions with some compounds, such as acid chlorides.
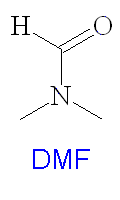 DMSO is widely used as a solvent in many organic syntheses and has several important industrial applications including polymer chemistry, pharmaceuticals and agrochemicals. It is a dipolar aprotic solvent, and has many similar properties to dimethylformamide (DMF) (see structure right). It is often used as the solvent for SN2 syntheses, and in the preparation of organometallics such as ferrocene. It is used as a rinsing agent in the electronics industry, and deuterated DMSO-d6 is used as a solvent for NMR spectroscopy. It is particularly good for this as it will dissolve a wide range of compounds, and does not interfere with the sample signals excessively. DMSO is used in antifreeze and it makes an effective paint stripper, which is safer than many other products such as nitromethane and dichloromethane. DMSO is used as a cryoprotectant for protecting human and other biological tissues when frozen for storage. DMSO also has several veterinary uses, such as as a liniment for horses, which relieves pain when rubbed onto the muscles.
DMSO is widely used as a solvent in many organic syntheses and has several important industrial applications including polymer chemistry, pharmaceuticals and agrochemicals. It is a dipolar aprotic solvent, and has many similar properties to dimethylformamide (DMF) (see structure right). It is often used as the solvent for SN2 syntheses, and in the preparation of organometallics such as ferrocene. It is used as a rinsing agent in the electronics industry, and deuterated DMSO-d6 is used as a solvent for NMR spectroscopy. It is particularly good for this as it will dissolve a wide range of compounds, and does not interfere with the sample signals excessively. DMSO is used in antifreeze and it makes an effective paint stripper, which is safer than many other products such as nitromethane and dichloromethane. DMSO is used as a cryoprotectant for protecting human and other biological tissues when frozen for storage. DMSO also has several veterinary uses, such as as a liniment for horses, which relieves pain when rubbed onto the muscles.
 According to Stanley Jacob (photo left) more than 40,000 articles on the chemistry of DMSO have appeared in scientific journals. 11,000 articles have been written on its medical and clinical implications, and in 125 countries throughout the world doctors prescribe it for a variety of ailments, including pain, inflammation, scleroderma, interstitial cystitis, arthritis and elevated intercranial pressure. Yet in the US, until 2016 DMSO had Food and Drug Administration (FDA) approval only for use as a preservative of organs for transplant and for interstitial cystitis, a bladder disease!
According to Stanley Jacob (photo left) more than 40,000 articles on the chemistry of DMSO have appeared in scientific journals. 11,000 articles have been written on its medical and clinical implications, and in 125 countries throughout the world doctors prescribe it for a variety of ailments, including pain, inflammation, scleroderma, interstitial cystitis, arthritis and elevated intercranial pressure. Yet in the US, until 2016 DMSO had Food and Drug Administration (FDA) approval only for use as a preservative of organs for transplant and for interstitial cystitis, a bladder disease!
After its clinical effects were discovered in 1961 it wasn't long before reporters, the pharmaceutical industry, and patients with a variety of medical complaints jumped on the news. However, since DMSO was widely available as a solvent and industrial chemical (rather than as a restricted drug), patients did not need to obtain a doctor's prescription to get hold of it. As a result, many patients began to dose themselves, often without knowing about the correct dosage or potential side-effects. As a result of these uncontrolled treatments, the FDA was unable to confirm that its experimentation and use were safe. The mainstream medical community became soured, and DMSO has been tainted with a bad reputation ever since.
Another problem with clinical testing is that its main side-effect, garlic-smelling breath, makes double-blind experiments difficult, because the patients (and doctors) can always tell who had been given DMSO and who had the placebo! The smell also puts off drug companies, who fear it would be hard to market. A bigger problem for the drug companies, however, is that because DMSO is a widespread industrial chemical and solvent, no company would be granted an exclusive patent for its medical use. Without potential profits, drug companies would not spend millions on the clinical testing required for FDA approval. The controversy really began in November 1965, when an Irish woman died of an allergic reaction after taking DMSO together with several other drugs. Although the precise cause of death was never determined, the press reported it to be DMSO. At around the same time laboratory animals that had been given doses of DMSO many times higher than would be given humans developed abnormalities in their eye lenses. Two months later the FDA closed down all clinical trials of DMSO in the United States, citing the woman's death and the negative findings from animal experiments as the reason. |
|
But 20 years and hundreds of laboratory and human studies later, no other deaths have been reported, nor have changes in the eyes of humans been reported or claimed. However, the FDA has refused seven applications to conduct clinical studies, and approved only 2, one for intersititial cystitis which was subsequently approved for prescription use in 1978, and another for the treatment of closed head injury.
In 2016 the EPA finally approved DMSO for medicinal use in the US, which at last opens up a lot of potential uses.
What could it be used for?Its main potential use is to ferry other drugs across membranes, including drugs such as morphine sulfate, penicillin, steroids, and cortisone. The fact that it can do so without opening the skin removes many problems associated with infections. It can also act as a local aneasthetic, reducing pain by blocking peripheral nerve fibres. Burns, cuts, and sprains have been treated with DMSO, and relief is reported to be almost immediate, lasting up to 6 hours. Because is an antioxidant, i.e. a scavenger of the free radicals that gather at the site of injury, DMSO can be used to reduce inflammation. Examples include relieving the symptoms of people with rheumatoid arthritis and chronic low-back inflammatory-type symptoms, silicon immune toxicity syndromes, or any kind of autoimmune process. DMSO may also be used as a clot-busting agent to help stroke patients, or to reduce intercranial pressure in patients with severe head trauma. It's been used to treat minor cuts and burns, soft tissue damage, local tissue death, skin ulcers, and burns, and may even delay the spread of certain types of cancer. Toxic shock, radiation sickness, and septicemia have also been suggested as possibly being responsive to DMSO. An intriguing possibility is that due to its antioxidant powers, DMSO could be used to mitigate some of the effects of ageing, but little work has been done to investigate this yet. |
|
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255644]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255644]