![]()

![]()
Roderick Edmonds
Eton College, UK
![]()
Molecule of the Month March 2021
![]()
Roderick Edmonds
Molecule of the Month March 2021
|
What's it like?The background to this page is white, rather than tastefully coloured, as titanium dioxide is normally a white powder - in fact, one of the "whitest" substances known. This is because:
|
 TiO2 is a white powder [Photo: Walkerma via Wikimedia Commons] |
A good question. Titanium is a transition metal, and many transition-metal compounds are intensely coloured. Hydrated copper(II)sulfate, for example, is blue because d-electrons in the hydrated Cu2+ ions are excited to a higher energy level by absorbing red light. The Ti4+ ion in TiO2 has no d-electrons to absorb visible wavelengths in this way. Importantly though, TiO2 can absorb ultraviolet light (see below), through excitation of electrons in its oxide ions.
 Rutile [Photo: Rob Lavinsky, iRocks.com, CC BY-SA 3.0, via Wikimedia Commons] |
Where does it come from?The mineral rutile (photo, left) contains titanium dioxide mixed with other materials such as oxides of iron, which give it a dark colour. Rutile is converted to pure titanium oxide by the “Chloride process”: The rutile is heated with carbon and chlorine, producing titanium(IV)chloride and also chlorides of iron. TiO2(s) + C(s) + 2Cl2(g) Titanium(IV)chloride, unlike titanium dioxide, is made of molecules rather than ions. Consequently, its melting and boiling points are low. It's a gas at the high temperature of the reaction, and condenses to a liquid at room temperature. It can then be separated easily from the iron chlorides which form solids. Reaction with oxygen produces pure titanium dioxide, and regenerates the chlorine. TiCl4 + O2 Compounds of metals usually have ionic giant structures, and hence high melting points. Only a few are made of molecules; aluminium chloride is another example which you may have met. |

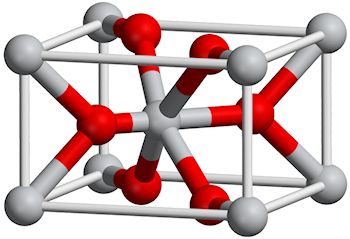 So TiO2 is an ionic crystal?
So TiO2 is an ionic crystal?Yes, although TiO2 can adopy many crystalline structures, the three major ones of which are rutile, anastase and Brookite. The one shown right is the rutile structure.
Look for something that's been painted. Titanium dioxide is the pigment in most white paints. An interesting fact is that the exterior of the Saturn V rocket was painted with titanium dioxide, which is why they always looked so dazzlingly white. In 2003 this allowed astronomers to determine that a near-Earth object that they'd just discovered (code named J002E3) was actually the jettisoned S-IVB stage from Apollo 12 and not an asteroid!
Titanium dioxide is used in coloured paints, too, and it's estimated that TiO2 is actually found in two-thirds of all pigments! For example, a blue paint will probably contain titanium dioxide together with a smaller amount of a blue pigment such as copper phthalocyanine. This sounds, and is, much more expensive than titanium dioxide, and if used on its own it would give a much darker blue than you'd probably want.
Not as a source of nutrition, but it's used to impart a white colour to many toothpastes . The ingredients label may identify it by its number in the international Colour Index; CI77891 or the E-number E171. There are some recent animal studies that suggest that prolonged ingestion of TiO2 can be carcnogenic; as a result France (but as yet no other countries) banned its use in foodstuffs from 2020.

A tube of toothpaste with the code for TiO2 circled.
Is it in sun cream too?A sunscreen cream to protect from solar ultraviolet radiation normally contains either an organic molecule which absorbs UV, or an inorganic pigment - either titanium dioxide or zinc oxide. Titanium dioxide particles about a micrometre (10-6 m) in size will both scatter and absorb UV light, so that little reaches the skin underneath. They also look like white paint, which may frighten the opposition in a cricket match but won't help you blend in on the beach. The solution is to reduce the size of the particles well below the wavelength of visible light, so they become transparent to it. They are still able to absorb and scatter UV, as shown in the picture below. |
 The Australian cricketer Andrew Symonds with white sunblock on his lips. [Photo: Privatemusings via Wikimedia Commons] |

TiO2 sheet with different sized particle, demonstrating how reducing particle size increases transparency.
[Photo: Reproduced with permission from a Tioxide (now Venator) brochure.]
There has been increasing concern in recent years about air pollution from nitrogen oxides, NO and NO2, collectively known as “NOx”. There is more about these pollutants and their effects in the MOTM article for December 2012.
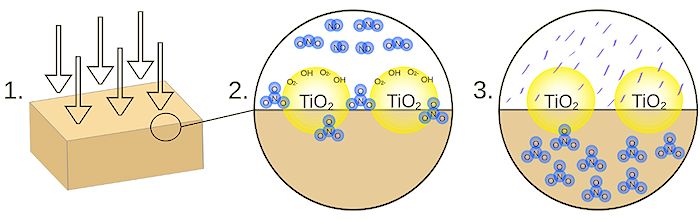
Operation of a Noxer Blocker.
[Diagram: Mark PEA via Wikimedia Commons]
A "Noxer Block" is a concrete block or paving stone with titanium dioxide nanoparticles embedded in its surface. The ability to absorb UV allows titanium dioxide to act as a photocatalyst, breaking down the pollutant gases NO and NO2 in the air. The diagram shows the steps involved.
|
Titanium dioxide is also used as a photocatalyst in self-cleaning windows. The glass is coated with titanium dioxide nanoparticles, and reactions caused by the absorbed UV break down large molecules in dirt to smaller products which wash away in the rain.
...And produce renewable fuel?Hydrogen is a possible replacement for fossil fuels, and is already used in buses in some cities around the world including London. Hydrogen has long been produced from hydrocarbons present in fossil fuels, but this rather defeats the object here. Electrolysis of water is a more sustainable alternative, assuming you don’t use electricity from a gas or coal-fired power station. A neater way is to make hydrogen from water using the energy of sunlight – green plants have been producing their fuels (sugars and starch) by photosynthesis since long before humans were around. The key to achieving this photodissociation of water is a suitable catalyst. Several catalyst systems are being researched, some of which involve titanium dioxide. Ultraviolet photons excite electrons from the “valence band” to the “conduction band” of titanium dioxide. In other words, electrons from the oxide ions are excited into empty orbitals of the Ti4+ ions. |
 Hydrogen-powered London bus. [Photo: Spsmiler via Wikimedia Commons] |
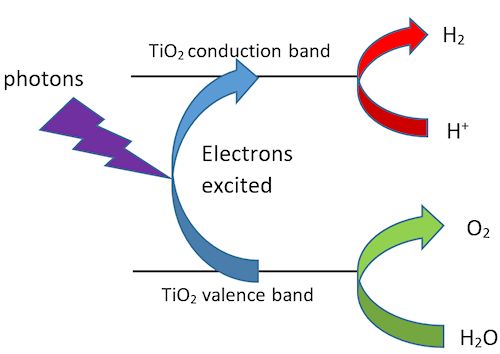 Photodissociation of water. |
This leaves gaps, or “holes” in the valence band where an electron ought to be. These are filled by the oxidation of water: 2H2O Meanwhile, the excited electrons reduce hydrogen ions: 4H+ + 4e- Hence the overall reaction is the conversion of water to hydrogen and oxygen, using energy from sunlight. 2H2O |
...And charge my phone?Titanium dioxide is also used in solar power generation as one component of a dye-sensitised solar cell (DSSC). A coloured dye is adsorbed onto the TiO2 so that the cell can use the energy of visible rather than just UV light. Unlike a silicon solar cell, a DSSC can easily be made in a flexible form. You might find this solar powered backpack useful for charging your phone or laptop on the way to school. |
 Solar-powered backpack. [Photo: Frommcc, CC BY-SA 3.0 via Wikimedia Commons] |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.13292063]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.13292063]