Acetyl Coenzyme AThe molecule that makes fats, or burns them.
Also available: HTML-only, JMol, and VRML versions.
|
|
Acetyl Coenzyme AThe molecule that makes fats, or burns them.
Also available: HTML-only, JMol, and VRML versions.
|
|
In the 1930's-early 1940's, four German-born biochemists, Fritz Lipmann, Hans Krebs, Feodor Lynen and Konrad Bloch, were investigating the mechanism by which glucose foods are metabolised in the body and turned into either fats for storage, or energy for immediate use. They worked independently, since three of them had fled from the Nazis to seek refuge in countries like Switzerland, the UK or US.
For their work, they were awarded the Nobel prize for Medicine, Fritz Lipmann and Hans Krebs in 1953, and Feodor Lynen and Konrad Bloch in 1964.
|
|
|
Between them, they found that one of the central molecules involved in this process is a coenzyme (a molecule that helps an enzyme), which was named coenzyme A (or CoA for short). The A stood for acetyl, since one of CoA's main jobs is to transfer two-carbon units in the form of acetyl between various biological molecules. It can be thought of as the body's 'delivery truck', since it transports its cargo of C2 along the roadways of blood vessels to the retail stores (muscles) where it's unloaded.
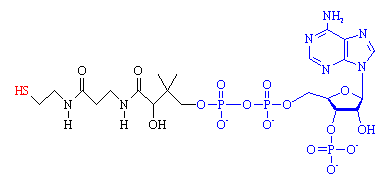
CoA is composed of two main parts, a long protein-like chain (shown in black in the figure), joined to adenosine diphosphate, ADP, (shown in blue) which is one of the molecules (along with ATP) used for energy storage. The important part of the molecule is at the end of the protein chain, which terminates in a sulph-hydryl (-SH) group (red). This group is highly reactive, and links to carboxylic acid molecules via a thioester bond. The most important acid is acetic acid, and when it is joined to CoA, the resulting compound is known as acetyl-CoA.
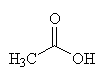 Acetic acid - the C2 carbon unit that joins to CoA.
Acetic acid - the C2 carbon unit that joins to CoA.
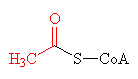 The thioester bond, joining together acetyl (shown in red) and CoA to make acetyl-CoA.
The thioester bond, joining together acetyl (shown in red) and CoA to make acetyl-CoA.
The thioester link, however, is very high energy bond, and therefore unstable. This means that the acetyl group can be easily transferred to other molecules, and so acetyl-CoA is used as a universal intermediate which provides the C2 fragment for numerous biochemical syntheses.
CoA is an extremely important biological molecule which is right at the hub of carbohydrate metabolism. This process is called the Citric Acid Cycle (or the tricarboxylic acid cycle, the TCA cycle, or the Krebs cycle - named after Hans Krebs) because citric acid (or more precisely, the citrate ion) is one of the key chemicals in the series of rections).
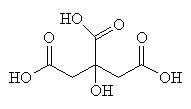 Citric acid - an important intermediate in the Citric Acid Cycle.
Citric acid - an important intermediate in the Citric Acid Cycle.
When you eat food, the various carbohydrates, fats and proteins are chopped up by enzymes in the liver and intestines into smaller units, usually C2 fragments. In the Citric Acid Cycle, these C2 fragments are reacted with CoA, to form acetyl-CoA. The acetyl residue is then transported via the blood to other molecules residing in cells all over the body, where it is released and, in the presence of O2, oxidised to carbon dioxide. This generates the energy that the cells require for biochemical processes, and the waste CO2 is transported in the bloodstream to the lungs and exhaled. The cycle also produces hydrogen atoms, which then continue in another series of biochemical reactions to produce adenosine triphosphate (ATP) and water. ATP is the molecule which stores energy, in the form of phosphate bonds, for later use. In the Citric Acid Cycle, one molecule of acetyl-CoA generates 12 ATPs. When the acetyl is released, CoA is regenerated, which returns to the Citric Acid Cycle to catalyse another reaction.
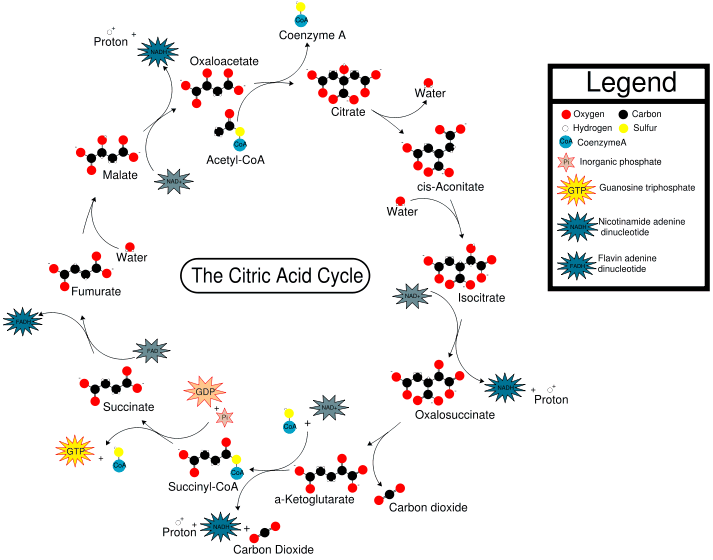
The Citric acid cycle (taken from Wikipedia)
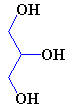
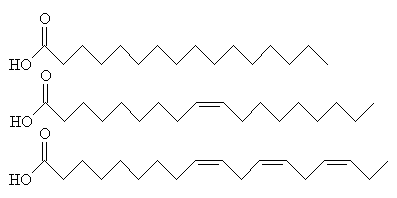
If the C2 unit is not to be oxidised to CO2 and the energy used immediately, another possibility is that it can be used to build important biological molecules. Acetyl-CoA is the starting point for the synthesis of fatty acids from carbohydrates. Fatty acids have carboxylic acid groups (-COOH) at one end, and a long alkane/alkene chain at the other. Examples include linoleic acid (a constituent of margarine), palmitic acid (from palm trees, and used as a constituent of napalm in WW2) and butyric acid (found in butter). Three of these fatty acids then join together with one molecule of glycerol to make triglyceride (also known as triacylglycerol) - and this forms the main constituent of vegetable oil and animal fats. The three fatty acids RCOOH, R'COOH and R"COOH can be all different, all the same, or only two the same, but what they have in common is that they were all synthesised using acetyl-CoA.
 +
+ 
![]()
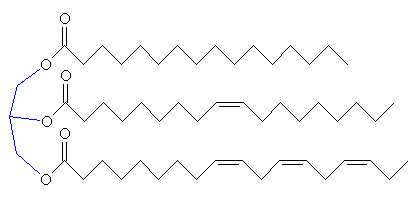
Glycerol (left) reacting with 3 fatty acids (top: palmitic acid, middle: oleic acid, bottom: α-linolenic acid) to form an unsaturated fat triglyceride.
 The chain lengths of the fatty acids in naturally occurring triglycerides can be from 5 to 28 carbon atoms. However, the natural fatty acids found in plants and animals are usually composed only of even numbers of carbon atoms, due to the way they are bio-synthesised from C2 units using acetyl-CoA. But bacteria possess the ability to synthesise odd- and branched-chain fatty acids. Therefore, the fat from ruminant animals (e.g. cows) contains significant proportions of branched-chain fatty acids, due to the action of bacteria in the gut.
The chain lengths of the fatty acids in naturally occurring triglycerides can be from 5 to 28 carbon atoms. However, the natural fatty acids found in plants and animals are usually composed only of even numbers of carbon atoms, due to the way they are bio-synthesised from C2 units using acetyl-CoA. But bacteria possess the ability to synthesise odd- and branched-chain fatty acids. Therefore, the fat from ruminant animals (e.g. cows) contains significant proportions of branched-chain fatty acids, due to the action of bacteria in the gut.

30 tonnes of triglycerides! The insulating blubber of this sperm whale
is composed mainly of fats made from triglycerides, each of which was made by acetyl-CoA.
...and Homer Simpson's beer belly (right) is made from triglycerides, too!
 In animals, both sugars (carbohydrates) and fats can be metabolised to produce energy, and acetyl-CoA is central to keeping the balance between these two. If the body takes in more sugar or fat than it needs, the excess is stored as fat, as described above. But when the body requires this energy again, the fat is metabolised with the help of acetyl-CoA. The stored triglycerides are cleaved to give 3 fatty acid chains and 1 glycerol molecule in a process called lipolysis. The glycerol is converted to glucose, and gives cells energy. And the 3 fatty acids provide an extra source of energy, as the long chains are cleaved 2-carbons at a time to form acetyl-CoA, which can then be fed into the Citric Acid Cycle. In cases of starvation, or where the person has a low carbohydrate diet (such as the Atkins Diet), this process can occur to excess. This can produce an unusually large amount of ketones from the breakdown of fatty acids to acetyl groups. In this situation, the person is said to be suffering from ketosis, and the ketones are excreted in the breath (and urine) giving the person's breath a characteristic sweet, fruity smell, that has been likened to the smell of nail varnish remover (acetone) or sometimes pear drops (ethyl ethanoate).
In animals, both sugars (carbohydrates) and fats can be metabolised to produce energy, and acetyl-CoA is central to keeping the balance between these two. If the body takes in more sugar or fat than it needs, the excess is stored as fat, as described above. But when the body requires this energy again, the fat is metabolised with the help of acetyl-CoA. The stored triglycerides are cleaved to give 3 fatty acid chains and 1 glycerol molecule in a process called lipolysis. The glycerol is converted to glucose, and gives cells energy. And the 3 fatty acids provide an extra source of energy, as the long chains are cleaved 2-carbons at a time to form acetyl-CoA, which can then be fed into the Citric Acid Cycle. In cases of starvation, or where the person has a low carbohydrate diet (such as the Atkins Diet), this process can occur to excess. This can produce an unusually large amount of ketones from the breakdown of fatty acids to acetyl groups. In this situation, the person is said to be suffering from ketosis, and the ketones are excreted in the breath (and urine) giving the person's breath a characteristic sweet, fruity smell, that has been likened to the smell of nail varnish remover (acetone) or sometimes pear drops (ethyl ethanoate).
![]()