![]()
Adenosine Triphosphate
(ATP)
The body's energy storage molecule.
![]()
Paul May
University of Bristol
![]()
Molecule of the Month - January 1998
(Update August 2016)
Also available: JSMol version.
![]()

|
Adenosine Triphosphate
|
 |
The acronym for adenosine triphosphate is ATP, which sounds like 80p (short for 80 pence).
All living things, plants and animals, require a continual supply of energy in order to function. The energy is used for all the processes which keep the organism alive. Some of these processes occur continually, such as the metabolism of foods, the synthesis of large, biologically important molecules, e.g. proteins and DNA, and the transport of molecules and ions throughout the organism. Other processes occur only at certain times, such as muscle contraction and other cellular movements. Animals obtain their energy by oxidation of the foods they’ve eaten, plants do so by trapping the sunlight using chlorophyll (see MOTM page for May 2000 on chlorophyll). However, before the energy can be used, it is first transformed into a form which the organism can handle easily. This special carrier of energy is the molecule ATP.
The key to how it works is in its structure. The ATP molecule is composed of three components. At the centre is a sugar molecule, ribose (the same sugar that forms the basis of DNA and RNA). Attached to one side of this is a base (a group consisting of linked rings of carbon and nitrogen atoms). In this case the base is adenine. When joined together, the sugar and base are known as adenosine. The other side of the sugar is attached to a string of three phosphate groups. These phosphates are crucial to the activity of ATP.
|
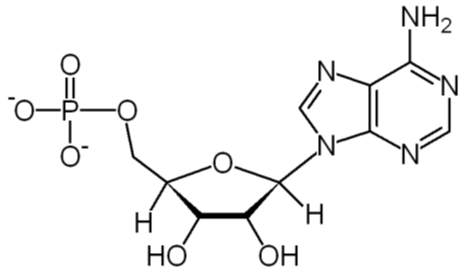
ATP works by losing the endmost phosphate group when instructed to do so by an enzyme. This reaction releases a lot of energy, which the organism can then use to build proteins, contract muscles, generate heat, etc. The reaction product has one less phosphate group and so is called adenosine diphosphate (ADP), and the liberated phosphate group either ends up in solution as orthophosphate (HPO4) or attached to another molecule such as an alcohol. Even more energy can be extracted by removing a second phosphate group to produce adenosine monophosphate (AMP).
ATP + H2O  ADP + HPO4 + lots of energy
ADP + HPO4 + lots of energy
ADP + H2O 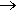 AMP + HPO4 + lots of energy
AMP + HPO4 + lots of energy
 |
 |
| ADP | AMP |
When the organism is resting and energy is not immediately needed, the reverse reaction takes place and the phosphate groups are reattached to the molecule, one at a time, using energy obtained from food or sunlight. Thus, the ATP molecule acts as a sort of rechargeable ‘chemical battery', storing energy when it is not needed, but able to release it instantly when the organism requires it. It has been calculated that the human body contains only 250 g of ATP at any one time, which contains roughly the equivalent of an AA battery. But it turns over more than its own weight in ATP in a day.
For long-term storage, i.e. days or years, surplus energy from food is used to synthesise long-chained fatty acids (see the MOTM page for April 2012 on lauric acid) and stored as fat distributed around the body, or as glycogen (a form of polymerised glucose, see the MOTM page for April 2007 on glucose) in the liver. When energy is required by the body for a particular process, say to make a muscle contract, the stored fat or glycogen is removed from storage by enzymes and transported in the blood to the cells in question. There, it undergoes oxidation, reacting with the oxygen delivered by haemoglobin in the bloodstream (see the MOTM page for Feb 2006 on Haemoglobin), to produce the waste products water and CO2, and releasing lots of energy. The energy converts ADP and AMP back to ATP (recharging the local ‘battery’), which is then used as the power source for the cellular process. So ATP is a very temporary energy store localised within each cell.
In plants, the long-term energy store is another polymerised form of sugar called starch (see MOTM page on glucose). In photosynthesis, the chlorophyll molecule traps energy from the sun and uses this to make ATP from ADP (see the MOTM page for May 2000 on chlorophyll). The ATP is then transported to other parts of the cell which break it back down into ADP, and use the released energy to turn carbon dioxide and water into glucose, releasing oxygen. Enzymes then polymerise the glucose into starch and it is stored for later use. Hydrolysis of the stored starch using enzymes (called amylases) allows the plant to extract the glucose and use the freed energy to make ATP, which can then be used as a local energy source in cells for use in various biological processes such as growth. Amylase enzymes are also present in human saliva and allow us to digest starch. Foods that contain a lot of starch but little sugar, such as rice and potatoes often taste slightly sweet because the amylase in saliva turns some of the starch into sugar as they are chewed.
We get the components from food, but these are metabolised into ATP in our body. The phosphate groups are the key ingredient, and these are part of what biologists call the Phosphorus Cycle. The fact that ATP is Nature's universal energy store explains why phosphates are a vital ingredient in the diets of all living things. Modern fertilizers often contain phosphorus compounds that have been extracted from animal bones. These compounds are used by plants to make ATP. Animals then eat the plants, metabolise the phosphates, and produce their own ATP. We also eat the plants and other animals, and convert their phosphorus into our own ATP. And when we die, our phosphorus goes back into the ecosystem to begin the cycle again...
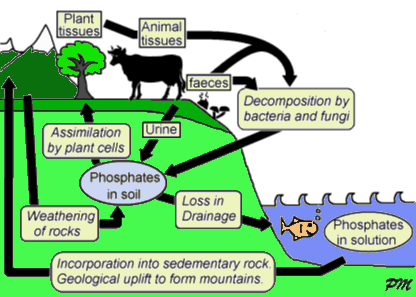
The Phosphorus Cycle in Nature.
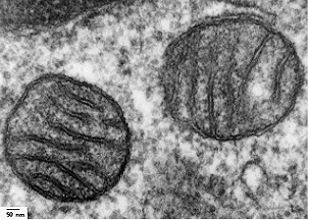
In animals, ATP is recycled in the mitochondria, which are organelles found within animals cells, and which can make up to 25% of the total volume of the cell. Mitochondria resemble smaller cells trapped within larger animal cells. They also contain their own DNA, which is different from the DNA found in the nucleus of the larger cells. This observation has led scientists to speculate that mitochondria were once separate cells (a sort of primitive bacteria) which had evolved the neat trick of synthesising ATP and thus storing energy. Other, larger, cells lacked this vital ability, but rather than waste a few million years evolving their own system to do so, they simply engulfed the mitochondrial cells and used them as internal power supplies. The mitochondria also benefit from this symbiosis, being protected from the dangers of the outside world, and nourished by a larger organism, for the small cost of some of their ATP.
 |
 |
| Transmission electron micrograph of mitochondria cells in lung tissue. |
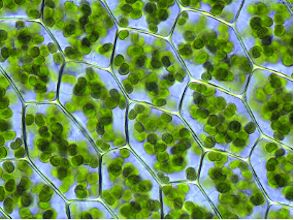
Rounded chloroplasts within the cells of thyme moss. |
In plants, the ATP is recycled in the cell membranes of chloroplasts (cells specialising in photosynthesis found in the leaves and surface of plants). Light energy from the sun pumps H+ ions across the cell membrane, and the potential difference this creates gives enzymes the power required to reattached phosphate groups to AMP or ADP. The energy stored in ATP is used to convert CO2 into glucose and larger organic molecules. The ancient ancestors of chloroplasts were single-celled algae called cyanobacteria, which are still around today. It is believed that chloroplasts evolved the ability to photosynthesise early on, and – just as with mitochondria – were then engulfed by larger cells. A symbiotic relationship then developed between the two cells until the point came where they both could no longer survive without each other.
This ‘endosymbiosis’ theory (‘endo’ meaning one partner is inside the other) for both mitochondria and chloroplasts is believed to be one of the key steps in evolution. The abundance of energy in the form of ATP given to multi-celled organisms by their new ‘internal power supplies’ allowed some of the organism’s other cells the freedom to specialise, and become light-sensitive (eye) cells, hairs for sensing or movement, antennae, etc., vastly increasing the complexity of life on Earth.
If you bought it as an aqueous solution of its disodium salt, the cost of a pint would be about $150,000 depending upon what concentration you wanted and what supplier you chose – significantly more than 80p, anyway!
The Nobel prize for Chemistry in 1997 has been shared by:
The prize was for the determination of the detailed mechanism by which ATP shuttles energy. The enzyme which makes ATP is called ATP synthase, or ATPase, and sits on the mitochondria in animal cells or chloroplasts in plant cells. Walker first determined the amino acid sequence of this enzyme, and then elaborated its 3-dimensional structure. Boyer showed that contrary to the previously accepted belief, the energy requiring step in making ATP is not the synthesis from ADP and phosphate, but the initial binding of the ADP and the phosphate to the enzyme. Skou was the first to show that this enzyme promoted ion transport through membranes, giving an explanation for nerve-cell ion transport as well as fundamental properties of all living cells. He later showed that the phosphate group that is ripped from ATP binds to the enzyme directly. This enzyme is capable of transporting sodium ions when phosphorylated like this, but potassium ions when it is not.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245498]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245498]